Recent in vivo studies show that preserving DNA sequences while altering methylation patterns can be safe and efficacious, encouraging clinical aspirations
Epigenome editing adds molecular markers or removes them from DNA, altering the expression of DNA-encoded proteins. It doesn’t alter the DNA sequence itself. Consequently, epigenetic editing can achieve fine control of gene expression, as opposed to simply introducing or eliminating a genetic function. That is, epigenome editing can provide a dimmer switch as opposed to an on/off switch. In addition, because epigenome editing doesn’t cut DNA, the risks posed by off-target effects are greatly reduced. Finally, in multiplexed applications, epigenome editing avoids introducing multiple cuts to the genome, raising the possibility of treating complex disorders without threatening genomic stability.
Several companies that have epigenome editing programs are cited in this article. Six companies are listed in Table, which includes selected details about financing and presentations at a recent conference (the 26th Annual Meeting of the American Society of Gene and Cell Therapy, or ASGCT 2023). Three of these companies are discussed in the main text. And one company is featured in a sidebar.
A miniaturist approach
Epic Bio, which is short for Epicrispr Biotechnologies, indicates that it has a platform that “combines a DNA-binding protein, a customized guide RNA, and any of a wide array of modulator proteins to create a powerful new type of genetic medicine.” The platform is called the Gene Expression Modulation System (GEMS), and it is designed to modulate gene expression both in vivo and ex vivo. One of the platform’s most interesting features is CasMINI, a DNA-binding molecule exclusively licensed to Epic Bio. According to the company, CasMINI is the smallest and most deliverable noncutting Cas protein to work in human cells.
Epic Bio was founded by Stanley Qi, PhD, an associate professor of bioengineering at Stanford University who is a named co-inventor on a CRISPR patent that reflects Nobel Prize–winning research and is held by the University of California. At Stanford, Qi developed the CasMINI, which continues to be refined at Epic Bio by Alexandra Collins de l’Hortet, PhD, the company’s head of therapeutics, and by Daniel Hart, PhD, the company’s head of technology development.
“We have also engineered, here at Epic, even smaller Cas proteins,” Hart says. “One of these Cas proteins is called CasOnyx. It is a proprietary molecule that we use in our lead program.” This program, EPI-321, focuses on treating facioscapulohumeral muscular dystrophy (FSHD).
Epic Bio is the only company addressing FSHD using an epigenetic targeting approach. Specifically, the company has designed EPI-321 to suppress expression of the DUX4 gene. “We aim to depress that gene by adding a methylation mark,” Collins de l’Hortet says. “Trying to target it at the RNA level with repeat administration is not trivial. Instead, by going after the epigenome and the DNA,” or the root cause, “we can be sure we suppress abnormal gene expression.”
Referring to an evaluation by Epic Bio of EPI-321’s performance, Collins de l’Hortet relates, “It has been well documented that we can suppress the expression of a gene. Nobody in the epigenetic field has been able to show long-lasting activation after the transient delivery of an epigenetic product, and we believe that we are the first ones to demonstrate that this is possible.”
“Based on the evidence of EPI-321’s ability to halt muscle cell death, and its favorable safety profile, we are committed to rapidly progressing this candidate to clinical studies,” she adds. Epic Bio plans to file an investigational new drug application with the FDA later this year, and the company is speaking with German officials about a clinical trial application for a small, six- to nine-person study. According to Epic Bio, a first-in-human clinical trial could be initiated for EPI-321 in 2024. “We haven’t confirmed exactly which trial sites will be selected,” says Amber Salzman, PhD, director and CEO of Epic Bio. “But we’re prioritizing those with excellence in FSHD.”
Silencing technology is golden
Besides attracting $125 million in Series A funding at its founding in 2021, Chroma Medicine secured $135 million in Series B funding in 2023. “We are pretty well capitalized right now,” says Vic Myer, PhD, president and chief scientific officer at Chroma Medicine and an advisor at Atlas Venture. “[It] gives us runway for well over 24 months.”
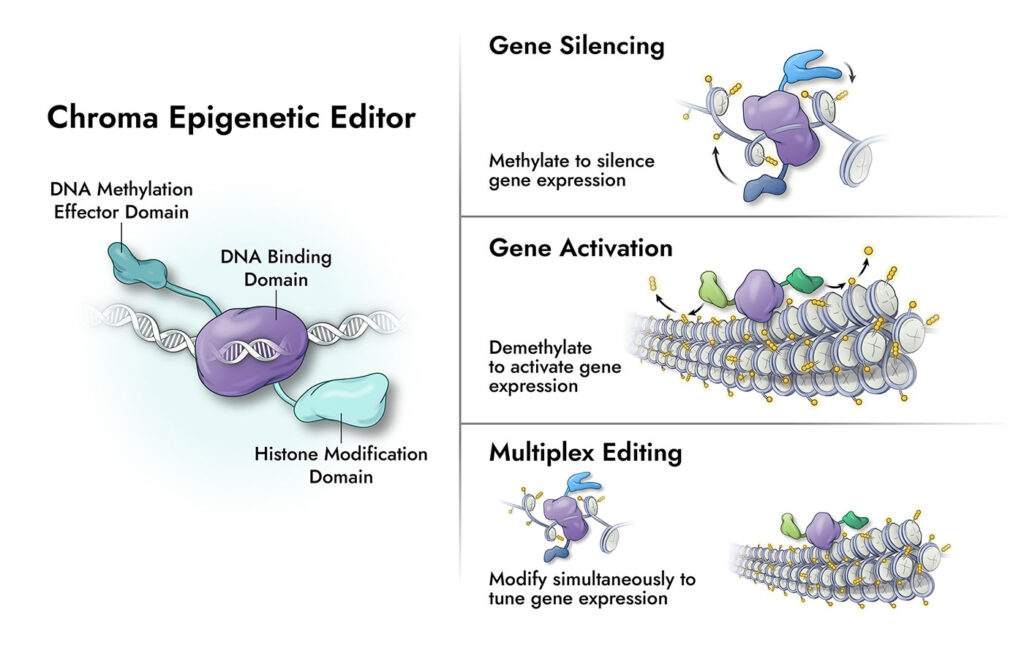
“Our focus is DNA methylation,” Myer notes. “We do this with a fusion protein that goes in and transiently adds or removes the methyl marks.” He adds that Chroma Medicine’s epigenetic editors have three parts: “First, there is a DNA-binding domain, which allows us to target specific locations of interest in the genome. Second, there is a methylation effector domain, either a DNA methyltransferase or DNA demethylase domain, to modify methyl marks—DNMT3A and DNMT3L, for example. Third, there is a transcriptional effector domain, which transiently represses the target gene. The advantage of this approach is that we see durable responses without cutting or nicking the DNA.”
“Two in vivo programs that are showing strong silencing in mice are on track for moving into nonhuman primate trials,” Myer asserts. He summarizes the clinical approach for both pipeline products as incorporating “mRNA plus a guide RNA plus a lipid nanoparticle.”
One of these early programs is focused on hepatitis B (HBV) infection. “HBV presents major challenges to patients around the globe that, from a genomic medicine perspective, are really difficult to address,” Myer relates. This is because HBV both integrates into the genome and persists in a special plasmid called an episome. When this episome is nicked or cut, Myer points out, “it drives new integration of the viral genome into the host genome because you are now creating a linear piece of DNA.”
“The beauty of the epigenetic approach,” Myer emphasizes, “is we can silence HBV regardless of whether it is episomal in nature or integrated into the DNA.”
Another early program is targeting the gene PCSK9. “It is a pretty well studied and well understood target in the field for hyperlipidemia,” Myer explains. “The loss of function of this gene does not have negative consequences, but what does happen is patients who have very low levels of PCSK9 also have very low levels of LDL-C and, therefore, a very low chance of developing cardiovascular disease.” According to Myer, this was a target Chroma Medicine chose because “we understand the on-target consequences for durably silencing that gene.”
At ASCGT 2023, Chroma Medicine revealed that its targeted epigenetic editor achieved 99% silencing of PCSK9, durable for at least five months post-dose. “We would reiterate PCSK9 has numerous advantages as a target, including the level of genetic and pharmacologic validation, the existence of preclinical models, and the ability to deliver to the tissue that is the main site of expression of the target,” Myer states. “Data from both of our groups reinforce the power of leveraging an endogenous mechanism for regulating gene expression to durably silence a therapeutically relevant target.”
Genetic tuning
In 2021, when Tune Therapeutics was launched, the company issued a press release that called its founders “world- leading researchers and venture builders.” The research side of that equation definitely includes two of the company’s three co-founders, namely, Fyodor Urnov, PhD, and Charles A. Gersbach, PhD, scientists who have decades of experience in gene regulation. The third co-founder, Akira Matsuno, is an accomplished biotech executive with a background in cell and gene therapies. He currently serves Tune as president and chief financial officer.
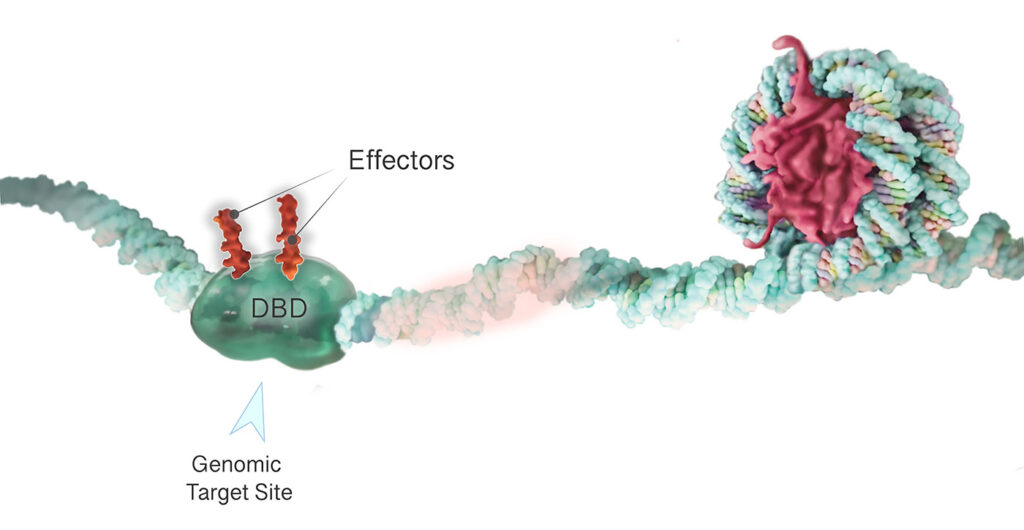
TEMPO consists of two switchable elements. One is a DNA-binding domain that enables the platform to bind to a tightly targeted region of the genome by recognizing specific DNA sequences situated near the target gene. The DNA-binding domain guides the protein to specific genomic target sites—enhancers, which act to enhance or increase gene activity, or repressors, which act to decrease gene activity. At the sites, effectors work to alter local epigenetic marks to either restrict or improve the ability of a cell’s transcription machinery to access and read the gene.

Akira Matsuno
Tune Therapeutics
The PCSK9 gene was deemed a suitable target by Tune, which saw the advantage of presenting a proof-of-concept study on a well-studied gene. “There’s been tons of data and programs that have targeted that gene, academic papers have described gene editing approaches for many years, and readouts are well understood,” Matsuno noted. By showcasing its technology with PSCK9, Tune expects to reassure investors.
“Safe and durable gene silencing following the transient delivery of an epi-repressor in primates represents a major milestone for the field,” maintains Derek Jantz, PhD, Tune’s chief scientific officer. “And it is a significant step toward enacting meaningful change for millions of patients suffering from common and chronic diseases.” The sentiment is seconded by Matsuno, who says, “Our next milestone is the clinic—with at least two undisclosed targets.”
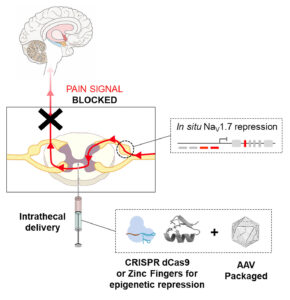
Pain Management through Epigenome Regulation
Navega Therapeutics is working to harness the precision of zinc finger nuclease– and CRISPR-Cas–based epigenome regulation to enable next-generation epigenome editing–based therapies. The company is focused on treating multiple chronic pain indications, including chemotherapy-induced peripheral neuropathy. Other indications in Navega’s pipeline include neurological and ophthalmic diseases.
“We employ epigenomic regulation tools through a non-immunogenic platform,” says Fernando Aleman, PhD, the company’s co-founder and chief science officer. “It is very exciting to see the validation of epigenome editing demonstrated with a hit-and-run approach. A single in vivo injection has provided months of pain relief in our animal models through the targeted repression of SCN9A (NaV1.7), a key gene involved in the propagation of pain signals.”
Navega focuses considerable energy on the “deployment of zinc fingers and de-immunized CRISPR-dCas9,” Aleman notes, “[alongside our] novel stealth AAV and circular RNA technology.” Novel zinc fingers are generated by leveraging artificial intelligence and machine learning technology. With this technology Navega intends to move quickly from targets to lead candidates. At present, Navega is working on two new undisclosed indications demonstrating in vivo multiplexing in epigenome editing.

Check out our AAV CDMO service to expedite your gene therapy research
PackGene is a CRO & CDMO technology company that specializes in packaging recombinant adeno-associated virus (rAAV) vectors. Since its establishment in 2014, PackGene has been a leader in the AAV vector CRO service field, providing tens of thousands of custom batches of AAV samples to customers in over 20 countries. PackGene offers a one-stop CMC solution for the early development, pre-clinical development, clinical trials, and drug approval of rAAV vector drugs for cell and gene therapy (CGT) companies that is fast, cost-effective, high-quality, and scalable. Additionally, the company provides compliant services for the GMP-scale production of AAVs and plasmids for pharmaceutical companies, utilizing five technology platforms, including the π-Alpha™ 293 cell AAV high-yield platform and the π-Omega™ plasmid high-yield platform. PackGene’s mission is to make gene therapy affordable and accelerate the launch of innovative gene drugs. The company aims to simplify the challenging aspects of gene therapy development and industrialization processes and provide stable, efficient, and economical rAAV Fast Services to accelerate gene and cell therapy development efforts from discovery phase to commercialization.
Related News
Exploring Tau Protein’s Role in Glaucoma: New Insights and Therapeutic Potential
Glaucoma, a chronic neurodegenerative disorder, leads to irreversible vision loss by damaging retinal ganglion cells (RGCs) and the optic nerve, often associated with increased intraocular pressure (IOP). Despite the benefits of IOP-lowering treatments, the underlying...
FDA-mandated CAR-T monitoring period could be halved, say researchers
In patients with diffuse large B-cell non-Hodgkin lymphoma (DLBCL), the two hallmark post-chimeric antigen receptor (CAR)-T therapy toxicities are extremely rare after two weeks, supporting a shorter, more flexible toxicity monitoring period, according to a study...
Ancestral CRISPR-Cas13 Ribonucleases Discovered: Implications for Genome Editing
In a pioneering study published in *Science*, a team of researchers led by Peter H. Yoon and Jennifer A. Doudna from the University of California, Berkeley, has made a remarkable discovery in the realm of CRISPR technology. The team has identified an ancestral clade...
KBI Biopharma Expands Manufacturing Contract with Global Pharmaceutical Company
KBI Biopharma Inc., a JSR Life Sciences company and global cGMP contract development and manufacturing organization (CDMO), has extended and expanded its manufacturing contract with a leading global pharmaceutical company. Originally initiated in 2020, the renewed...
Related Services

Plasmids GMP Services
Multiple scales & grade of solutions of various kind of plasmids suitable for multiple treatments in a fast and cost effective way.
READ MORE

AAV GMP Services
Ranging from small-scale AAV production, to large-scale AAV cGMP manufacturing for animal studies.
READ MORE

Technology Platforms
PackGene’s proprietary π-Alpha™ 293 AAV High-yield Platform increases AAV production by 3 to 8 times that of traditional platforms.
READ MORE

