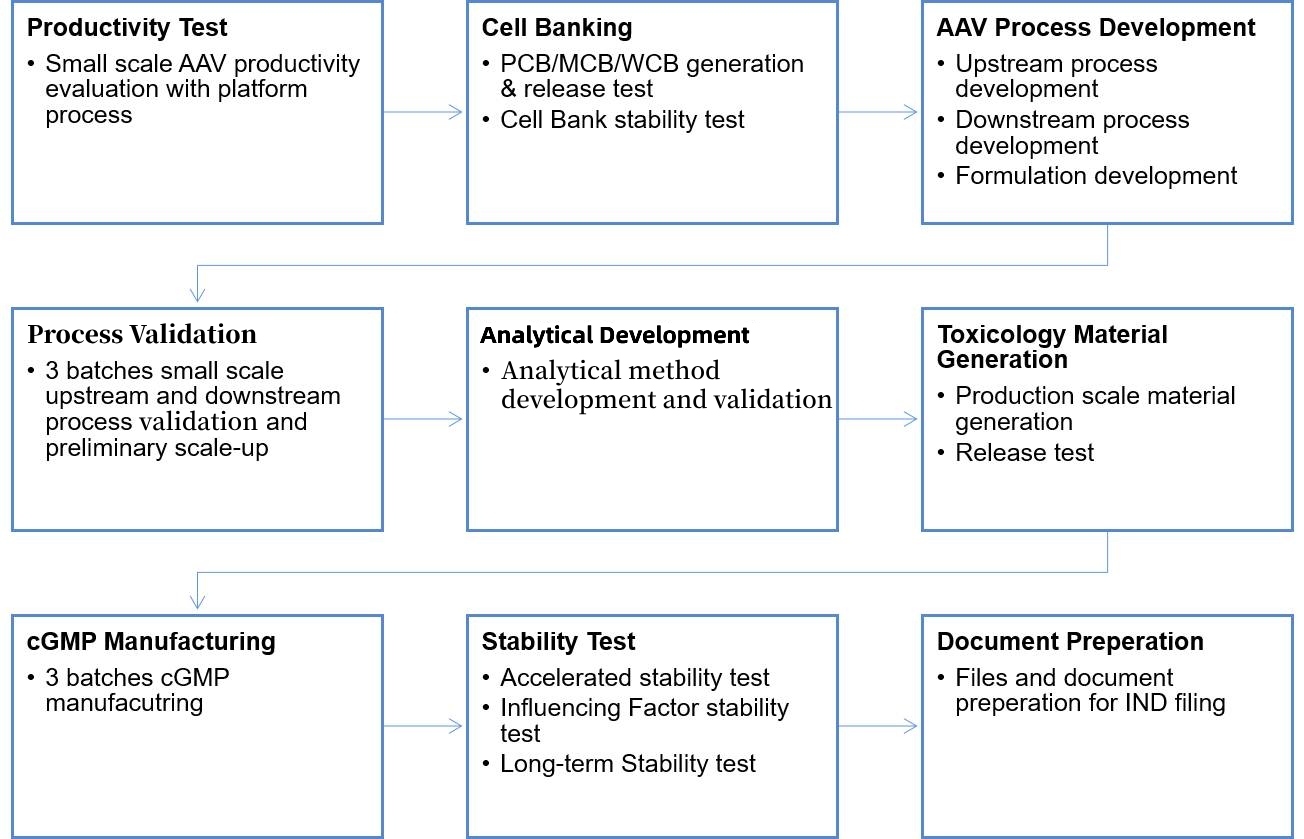
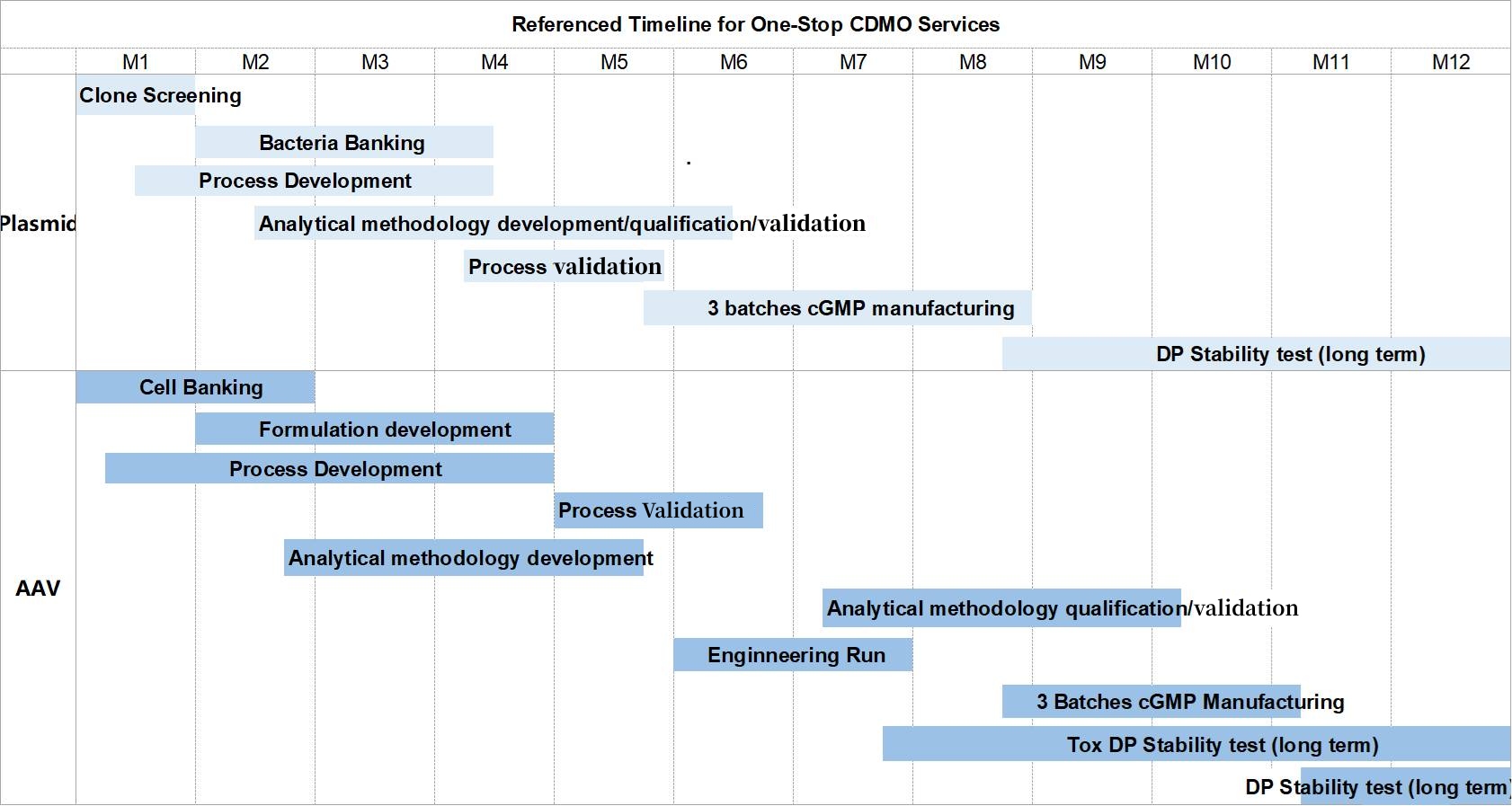
AAV are the most widely used vectors for in vivo gene delivery due to their targeting specificity and exceptional safety when compared with other vectors. PackGene provides one-stop solution services for AAV IND application including cell banking, process development, analytical development, AAV manufacturing, and stability tests with filing document preparation. Our professional AAV teams and technological superiority will deliver your IND project with first-class quality and speed.
Productivity
Both Suspension and adherent culture process available. Four independent cGMP standard production lines with up to 500L single-batch production scale and overall productivity up to 2000L
Advanced equipment
Single-use technology applied one both upstream & downstream. Fill & Finish under VHP isolator. ddPCR for titer measurements, Analytical ultra-centrifugation for e/f ratio measurements available.
Quality
Our proprietary platforms, single use technologies, and segregated production facilities ensure the delivery of deliverables without cross-contamination
Flexibility
Multiple scales and grades of production make it possible for us to complete your project with high efficacy and reasonable price.
Professional
PackGene has a professional AAV production team with more than 10 years of experience.
Resources
Are pH measurements required, and is a large amount of sample wasted to carry out pH measurements?
Measurement of pH is a mandatory for the release of rAAV Fast Service deliverables. A micro pH electrode may be used to save sample and thus the required sample volume to perform pH measurements is only ~15uL-100uL.
What is loading?
In accordance with the Pharmacopoeia General Rules 0942, we use the minimum filling quantity inspection method for detecting sample loading quantity.
How to interpret A260/A280 value?
A260/A280 is the ratio of sample absorbance measured at wavelengths of 260nm and 280nm. This measure is commonly thought to represent the ratio of DNA to protein in a sample. For rAAV, A260/A280 can used as a measure of the full to empty shell rate and to identify protein contamination. Low A260/A280 levels may suggest that the empty shell rate is high. Alternatively, high A260/A280 may suggest that the sample has been contaminated with proteins that are not incorporated into the AAV capsid shell. The greatest advantages of this measure are its convenience and speed.
What tests are performed to differentiate rAAV capsid proteins from specific protein impurities?
SDS-PAGE is used to identify rAAV capsid proteins. In addition, SDS-PAGE can be used to directly identify specific protein impurities including the presence of host proteins, BSA, or degraded AAV capsid proteins.
Related Services

Plasmids GMP Services
Multiple scales & grade of solutions of various kind of plasmids suitable for multiple treatments in a fast and cost effective way
READ MORE

AAV GMP Services
Ranging from small-scale AAV production, to large-scale AAV cGMP manufacturing for animal studies
READ MORE

Technology Platforms
PackGene’s proprietary π-Alpha 293 AAV High-yield Platform increases AAV production by 3 to 8 times that of traditional platforms.
READ MORE