The study was published in Scientific Reports.
Hurler syndrome, also known as mucopolysaccharidosis type I (MPS I), is a genetic disorder affecting newborns that leads to severe cognitive deficiencies and severe physical abnormalities. Genetic mutations disrupt synthesis of an essential lysosomal enzyme IDUA, resulting in progressive brain damage. Death occurs by 10 years of age. Current treatments are inadequate—bone marrow transplants are dangerous and lifetime enzyme replacement fails to prevent progressive brain damage
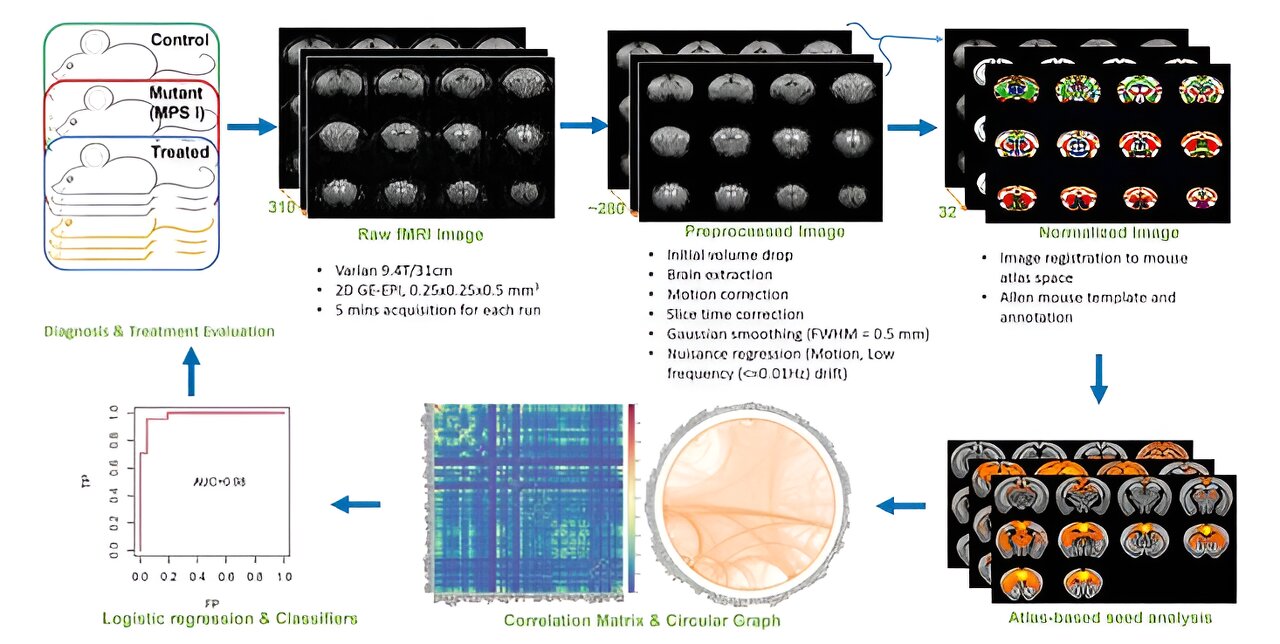
U of M researchers evaluated a new form of gene therapy invented at the University of Minnesota—the PS gene-editing system—in mice with Hurler syndrome. This approach created very high, continuous levels of normal enzymes in the liver that can enter the brain via the circulatory system. Using high resolution resting-state functional MRI (rs-fMRI)—a safe, noninvasive and whole-brain activity imaging tool for diagnosis and post-treatment evaluation—investigators first identified neural networks that were disrupted. Next, they assessed the extent to which brain functions and connectivity were restored following the gene therapy.
The investigators observed the new approach of PS gene-editing produced normal enzymes from the liver that were able to sustain normal connections within specific neural networks. The technology needed for high resolution rs-fMRI brain connectome imaging was developed by Wei Zhu, a graduate student in the University’s Center for Magnetic Resonance Research.
Walter Low, a co-senior author and professor in the U of M Medical School, referred to the study as a breakthrough, noting, “This is the first demonstration of a gene therapy that has corrected a neurological disorder resulting in the restoration of brain connectivity as confirmed by rs-fMRI.”
“A similar rs-fMRI approach as applied in this preclinical study should be translatable to the clinical setting and patients, especially for those with genetic brain disorders, and for examining the efficacy of brain network restoration and function after gene treatment,” said Wei Chen, a co-senior author and professor in the U of M Medical School and Center for Magnetic Resonance Research.
“The aeonic production of normal IDUA in the liver of mice with Hurler syndrome and the ability to traffic enzymes across the blood-brain barrier to correct abnormalities in the brain is a significant achievement,” added Chester Whitley, a co-author and professor in the U of M Medical School.
“This new approach will enable the monitoring of brain connectivity in other lysosomal disorders that affect brain function following gene-editing,” stated Perry Hackett, a co-author and professor in the College of Biological Sciences.
Other participants in this study include Lin Zhang, an associate professor in the School of Public Health; Ying Zhang, an informatics analyst in the Minnesota Supercomputing Institute; Xiao-Hong Zhu, a professor in the U of M Medical School and Center for Magnetic Resonance Research; Isaac Clark, a graduate student in the Biomedical Engineering Program; and Li Ou, a former faculty member in the U of M Medical School.

Check out our AAV CDMO service to expedite your gene therapy research
PackGene is a CRO & CDMO technology company that specializes in packaging recombinant adeno-associated virus (rAAV) vectors. Since its establishment in 2014, PackGene has been a leader in the AAV vector CRO service field, providing tens of thousands of custom batches of AAV samples to customers in over 20 countries. PackGene offers a one-stop CMC solution for the early development, pre-clinical development, clinical trials, and drug approval of rAAV vector drugs for cell and gene therapy (CGT) companies that is fast, cost-effective, high-quality, and scalable. Additionally, the company provides compliant services for the GMP-scale production of AAVs and plasmids for pharmaceutical companies, utilizing five technology platforms, including the π-Alpha™ 293 cell AAV high-yield platform and the π-Omega™ plasmid high-yield platform. PackGene’s mission is to make gene therapy affordable and accelerate the launch of innovative gene drugs. The company aims to simplify the challenging aspects of gene therapy development and industrialization processes and provide stable, efficient, and economical rAAV Fast Services to accelerate gene and cell therapy development efforts from discovery phase to commercialization.
Related News
Exploring Tau Protein’s Role in Glaucoma: New Insights and Therapeutic Potential
Glaucoma, a chronic neurodegenerative disorder, leads to irreversible vision loss by damaging retinal ganglion cells (RGCs) and the optic nerve, often associated with increased intraocular pressure (IOP). Despite the benefits of IOP-lowering treatments, the underlying...
FDA-mandated CAR-T monitoring period could be halved, say researchers
In patients with diffuse large B-cell non-Hodgkin lymphoma (DLBCL), the two hallmark post-chimeric antigen receptor (CAR)-T therapy toxicities are extremely rare after two weeks, supporting a shorter, more flexible toxicity monitoring period, according to a study...
Ancestral CRISPR-Cas13 Ribonucleases Discovered: Implications for Genome Editing
In a pioneering study published in *Science*, a team of researchers led by Peter H. Yoon and Jennifer A. Doudna from the University of California, Berkeley, has made a remarkable discovery in the realm of CRISPR technology. The team has identified an ancestral clade...
KBI Biopharma Expands Manufacturing Contract with Global Pharmaceutical Company
KBI Biopharma Inc., a JSR Life Sciences company and global cGMP contract development and manufacturing organization (CDMO), has extended and expanded its manufacturing contract with a leading global pharmaceutical company. Originally initiated in 2020, the renewed...
Related Services

Plasmids GMP Services
Multiple scales & grade of solutions of various kind of plasmids suitable for multiple treatments in a fast and cost effective way.
READ MORE

AAV GMP Services
Ranging from small-scale AAV production, to large-scale AAV cGMP manufacturing for animal studies.
READ MORE

Technology Platforms
PackGene’s proprietary π-Alpha™ 293 AAV High-yield Platform increases AAV production by 3 to 8 times that of traditional platforms.
READ MORE

