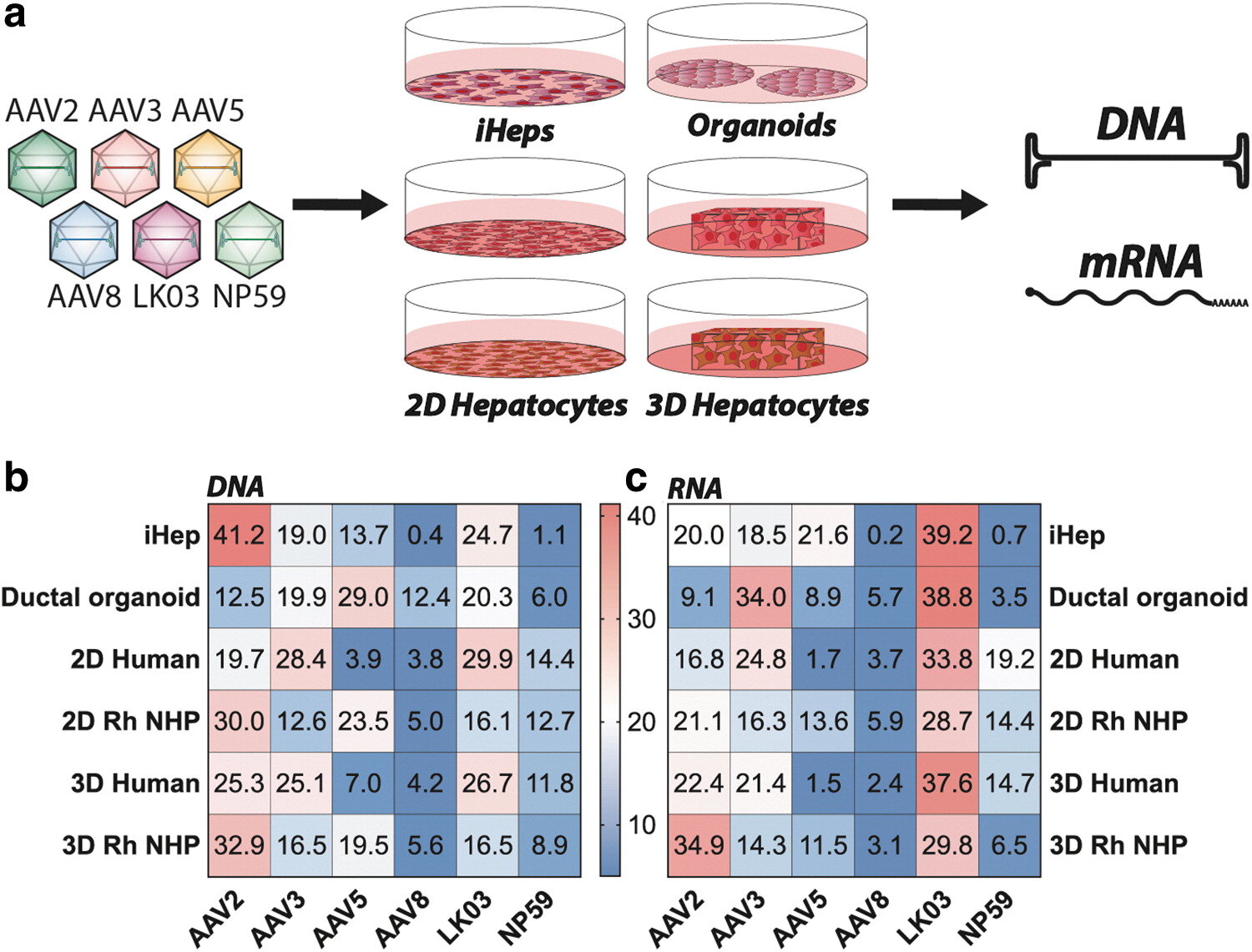
A new study reports the findings of the functional evaluation of six AAV vectors in 12 preclinical models of the human liver. The study, which aimed to uncover which combination of models is the most relevant for the identification of an AAV capsid variant for safe and efficient gene therapy to primary human hepatocytes, is published in Human Gene Therapy.
Leszek Lisowski, from The University of Sydney, and co-authors, note that robust, biologically- and clinically-predictive preclinical models of the safety and efficacy of liver-targeted AAV-based gene therapy are lacking. The investigators compared AAV-based gene transfer efficiency targeting the liver in 12 frequently used preclinical models, including in vitro models, such as hepatic cell lines, human-induced pluripotent stem cell (hiPSC)-derived hepatocytes, and adult stem cell-derived hepaltic organoids.
They focused primarily on ex vivo models, such as 2D and 3D primary non-human primates (NHP) and human hepatocytes cultures, and in vivo models, including murine and human hepatocytes in xenograft mice and NHPs.
“Even though a perfectly predictive preclinical model does not exist, our study shows that each model provides a unique insight into the vector function,” stated the investigators.
“Based on our results and the fact that each model brings a unique perspective that adds to the overall functional evaluation of AAV vectors, we propose that multiple models should be used to paint a more complete picture and help us make the most informed decision as to which vector should be used in each clinical application.”
“This study represents a highly innovative approach to combining data from multiple different experimental systems into an overall predictive model,” says Editor-in-Chief Terence R. Flotte, MD, Celia and Isaac Haidak Professor of Medical Education and Dean, Provost, and Executive Deputy Chancellor, University of Massachusetts Chan Medical School.
More information: Adrian Westhaus et al, Assessment of Pre-Clinical Liver Models Based on Their Ability to Predict the Liver-Tropism of Adeno-Associated Virus Vectors, Human Gene Therapy (2023). DOI: 10.1089/hum.2022.188
PackGene is a CRO & CDMO technology company that specializes in packaging recombinant adeno-associated virus (rAAV) vectors. Since its establishment in 2014, PackGene has been a leader in the AAV vector CRO service field, providing tens of thousands of custom batches of AAV samples to customers in over 20 countries. PackGene offers a one-stop CMC solution for the early development, pre-clinical development, clinical trials, and drug approval of rAAV vector drugs for cell and gene therapy (CGT) companies that is fast, cost-effective, high-quality, and scalable. Additionally, the company provides compliant services for the GMP-scale production of AAVs and plasmids for pharmaceutical companies, utilizing five technology platforms, including the π-Alpha™ 293 cell AAV high-yield platform and the π-Omega™ plasmid high-yield platform. PackGene’s mission is to make gene therapy affordable and accelerate the launch of innovative gene drugs. The company aims to simplify the challenging aspects of gene therapy development and industrialization processes and provide stable, efficient, and economical rAAV Fast Services to accelerate gene and cell therapy development efforts from discovery phase to commercialization.
Related News
Exploring Tau Protein’s Role in Glaucoma: New Insights and Therapeutic Potential
Glaucoma, a chronic neurodegenerative disorder, leads to irreversible vision loss by damaging retinal ganglion cells (RGCs) and the optic nerve, often associated with increased intraocular pressure (IOP). Despite the benefits of IOP-lowering treatments, the underlying...
FDA-mandated CAR-T monitoring period could be halved, say researchers
In patients with diffuse large B-cell non-Hodgkin lymphoma (DLBCL), the two hallmark post-chimeric antigen receptor (CAR)-T therapy toxicities are extremely rare after two weeks, supporting a shorter, more flexible toxicity monitoring period, according to a study...
Ancestral CRISPR-Cas13 Ribonucleases Discovered: Implications for Genome Editing
In a pioneering study published in *Science*, a team of researchers led by Peter H. Yoon and Jennifer A. Doudna from the University of California, Berkeley, has made a remarkable discovery in the realm of CRISPR technology. The team has identified an ancestral clade...
KBI Biopharma Expands Manufacturing Contract with Global Pharmaceutical Company
KBI Biopharma Inc., a JSR Life Sciences company and global cGMP contract development and manufacturing organization (CDMO), has extended and expanded its manufacturing contract with a leading global pharmaceutical company. Originally initiated in 2020, the renewed...
Related Services

AAV Packaging - NHP Grade
READ MORE

Off the Shelf AAV Products
We offer a wide catalogue of pre-made AAVs for a multitude of research needs.
READ MORE

AAV Capsid Engineering
Proven technology paving your path to effective therapies for cancer or genetic disorder
READ MORE

