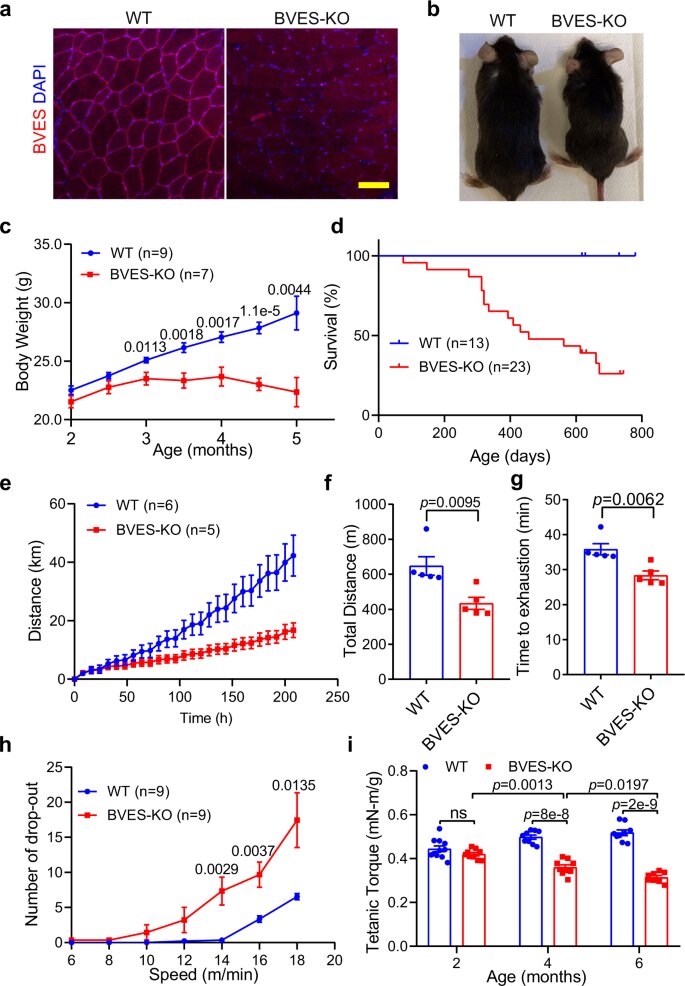
BVES disruption compromises the body weight gain and muscle function in mice. All animal experiments were performed in WT and BVES-KO male mice with the C57BL/6N genetic background. a Immunofluorescence images of GA muscles in WT and BVES-KO mice (4 months of age) stained with the antibody against BVES and DAPI. Scale bar: 100 µm. (n = 4 per genotype). b Representative image of WT and BVES-KO male littermates at 4 months of age. c, Body weight gain of male BVES-KO and age/sex-matched WT mice from two to five months of age. Two-tailed paired Student’s t test. d Kaplan–Meier survival curve of WT and BVES-KO male mice. e Voluntary wheel running of BVES-KO and age-matched WT male mice (4 months of age). f, g Endurance capacity test performed by treadmill running showing running distance (f) and time to exhaustion (g) in BVES-KO (n = 5) and WT (n = 5) male mice (4 months of age). Two-tailed unpaired Student’s t test. h The number of dropouts to test the capacity of recovery from muscle injury on the treadmill in BVES-KO and WT male mice (6 months of age). Two-tailed paired Student’s t test. i Tetanic torque measurements of the posterior compartment muscles of BVES-KO and WT male mice in age-dependent manner (2-month age: WT (n = 11), BVES-KO (n = 9); 4-month age: WT (n = 9), BVES-KO (n = 9); 6-month age: WT (n = 9), BVES-KO (n = 8)). ns indicates no significant difference. Two-way ANONA with Tukey’s multiple comparisons test. Data are mean ± SEM. Source data are provided as a Source Data file. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-37496-8
A study by Indiana University School of Medicine researchers sheds new light on the development and treatment of a rare form of muscular dystrophy. The study’s findings were recently published in Nature Communications.
Muscular dystrophies are a group of muscle diseases caused by gene mutations. There are several major varieties, including limb-girdle muscular dystrophy. According to the Centers for Disease Control, about 2 in 100,000 people are affected by various subtypes of limb-girdle muscular dystrophy which can develop at any age.
“Being a relatively new form of muscular dystrophy, very little is known about the development of limb-girdle muscular dystrophy type 25 and there is no treatment available for patients,” said Renzhi Han, Ph.D., professor of pediatrics at the Herman B Wells Center for Pediatric Research and the senior author of the article. “Our study’s main goal was to understand the molecular pathogenesis of muscular dystrophy caused by genetic mutations in BVES and develop a therapeutic treatment for this form of the disease.“
The study investigated the role of blood vessel epicardial substance (BVES) protein in the development of muscular dystrophy and atrophy. The researchers found that the study’s model engineered to lack BVES showed symptoms similar to those observed in humans. Further investigation showed that BVES interacts with an enzyme called ADCY9, inhibiting its activity in synthesizing cyclic AMP, an important signaling molecule in many biological processes.
The study also found the absence of BVES resulted in overactive ubiquitin proteasome degradation. Importantly, bortezomib, a specific inhibitor of ubiquitin proteasome degradation approved by the United States Food and Drug Administration for the treatment of certain cancers, significantly ameliorates the pathology of BVES-knockout skeletal muscle. Overall, these findings suggest that BVES may play a crucial role in maintaining muscle health and pharmacological treatments inhibiting proteasome degradation may be effective in alleviating BVES-deficient muscular dystrophy.
“Understanding the underlying causes of muscular dystrophy gets us one step closer to finding cures for patients,” said Haiwen Li, Ph.D., postdoctoral fellow and co-author of the published study. “Our research sheds new light on therapeutic treatment options for patients with BVES mutations and it will lead to additional important investigations to help other diseases.”
In addition to muscular dystrophy, BVES mutations are also linked to cardiac arrhythmia and cancer. Looking ahead, the researchers plan to investigate the roles of gene mutations in other health issues. They previously performed a preclinical study that demonstrated adeno-associated virus-BVES can improve muscle pathology and function in a mouse model with limb-girdle muscular dystrophy type 25. Results from that research were published in Molecular Therapy.
PackGene is a CRO & CDMO technology company that specializes in packaging recombinant adeno-associated virus (rAAV) vectors. Since its establishment in 2014, PackGene has been a leader in the AAV vector CRO service field, providing tens of thousands of custom batches of AAV samples to customers in over 20 countries. PackGene offers a one-stop CMC solution for the early development, pre-clinical development, clinical trials, and drug approval of rAAV vector drugs for cell and gene therapy (CGT) companies that is fast, cost-effective, high-quality, and scalable. Additionally, the company provides compliant services for the GMP-scale production of AAVs and plasmids for pharmaceutical companies, utilizing five technology platforms, including the π-Alpha™ 293 cell AAV high-yield platform and the π-Omega™ plasmid high-yield platform. PackGene’s mission is to make gene therapy affordable and accelerate the launch of innovative gene drugs. The company aims to simplify the challenging aspects of gene therapy development and industrialization processes and provide stable, efficient, and economical rAAV Fast Services to accelerate gene and cell therapy development efforts from discovery phase to commercialization.
Related News
Exploring Tau Protein’s Role in Glaucoma: New Insights and Therapeutic Potential
Glaucoma, a chronic neurodegenerative disorder, leads to irreversible vision loss by damaging retinal ganglion cells (RGCs) and the optic nerve, often associated with increased intraocular pressure (IOP). Despite the benefits of IOP-lowering treatments, the underlying...
FDA-mandated CAR-T monitoring period could be halved, say researchers
In patients with diffuse large B-cell non-Hodgkin lymphoma (DLBCL), the two hallmark post-chimeric antigen receptor (CAR)-T therapy toxicities are extremely rare after two weeks, supporting a shorter, more flexible toxicity monitoring period, according to a study...
Ancestral CRISPR-Cas13 Ribonucleases Discovered: Implications for Genome Editing
In a pioneering study published in *Science*, a team of researchers led by Peter H. Yoon and Jennifer A. Doudna from the University of California, Berkeley, has made a remarkable discovery in the realm of CRISPR technology. The team has identified an ancestral clade...
KBI Biopharma Expands Manufacturing Contract with Global Pharmaceutical Company
KBI Biopharma Inc., a JSR Life Sciences company and global cGMP contract development and manufacturing organization (CDMO), has extended and expanded its manufacturing contract with a leading global pharmaceutical company. Originally initiated in 2020, the renewed...
Related Services

AAV Packaging Services
Ranging from pilot to industrial-scale AAV packaging for both in vitro and in vivo studies.
READ MORE

Off the Shelf AAV Products
We offer a wide catalogue of pre-made AAVs for a multitude of research needs.
READ MORE

AAV Capsid Engineering
Proven technology paving your path to effective therapies for cancer or genetic disorder
READ MORE

