The hematopoietic system is a complex tissue characterized by multiple layers of stem cells, progenitor cells, and mature blood cells. Operating under normal conditions, it ensures a continuous and stable blood supply throughout the body.
Genetic dysregulation of gene expression in hematopoietic stem cells (HSCs) hinders their normal differentiation into myeloid cells, resulting in the accumulation of immature blast cells and the onset of acute myeloid leukemia (AML).
With the current intensive chemotherapeutic regimen, which has been in use for over 50 years, 50% of patients with AML experience a relapse within one year, while 80% of patients experience a relapse within five years. Thus, there is an unmet need for novel targeted therapies which can improve patient prognosis and survival.
To this end, powerful analytical approaches have helped unravel the mysteries underlying functional changes inside cells, such as post-transcriptional modifications of RNAs, which play an important role in several biological processes. These post-transcriptional RNA modifications, which involve reversible addition or removal of chemical groups to the RNA structure, have been implicated in various diseases, including cancer.
In a recent review article, researchers led by Professor Xiaoshuang Wang, affiliated with the Chinese Academy of Medical Sciences and Peking Union Medical College, China, have shed light on the role of RNA methylation and demethylation in normal hematopoiesis and AML pathogenesis as novel therapeutic agents.
“Although several dozens of RNA modification regulators have been identified, only a few of them may have the potential to serve as therapeutic targets in the clinic, as their underlying mechanisms are not systematically studied,” explains Prof. Wang, the corresponding author of the article which was published in the Chinese Medical Journal.
Among the more than 170 post-transcriptional RNA modifications identified over the past several decades, N6-methyladenosine (m6A) and 7-methylguanine (m7G) are the most prevalent and well-characterized modifications in eukaryotic mRNA. Regulators of m6A and m7G RNA methylation have been implicated in the pathogenesis of fatty liver disease, heart disease, and several cancers and, therefore, hold promise as potential therapeutic targets.
The regulatory machinery comprises protein complexes known as RNA “readers” or binding proteins, which recognize and bind to RNA residues. Additionally, there are “writers” or methyltransferases that add methyl groups and “erasers” or demethylases that remove methyl groups.
The process of DNA methylation and demethylation is a complex and tightly regulated interplay between different proteins. m6A modifications play a key role in normal HSC differentiation, self-renewal, and DNA damage response. On the contrary, dysregulation of m6A modification has been associated with cancer initiation, progression, and prognosis.
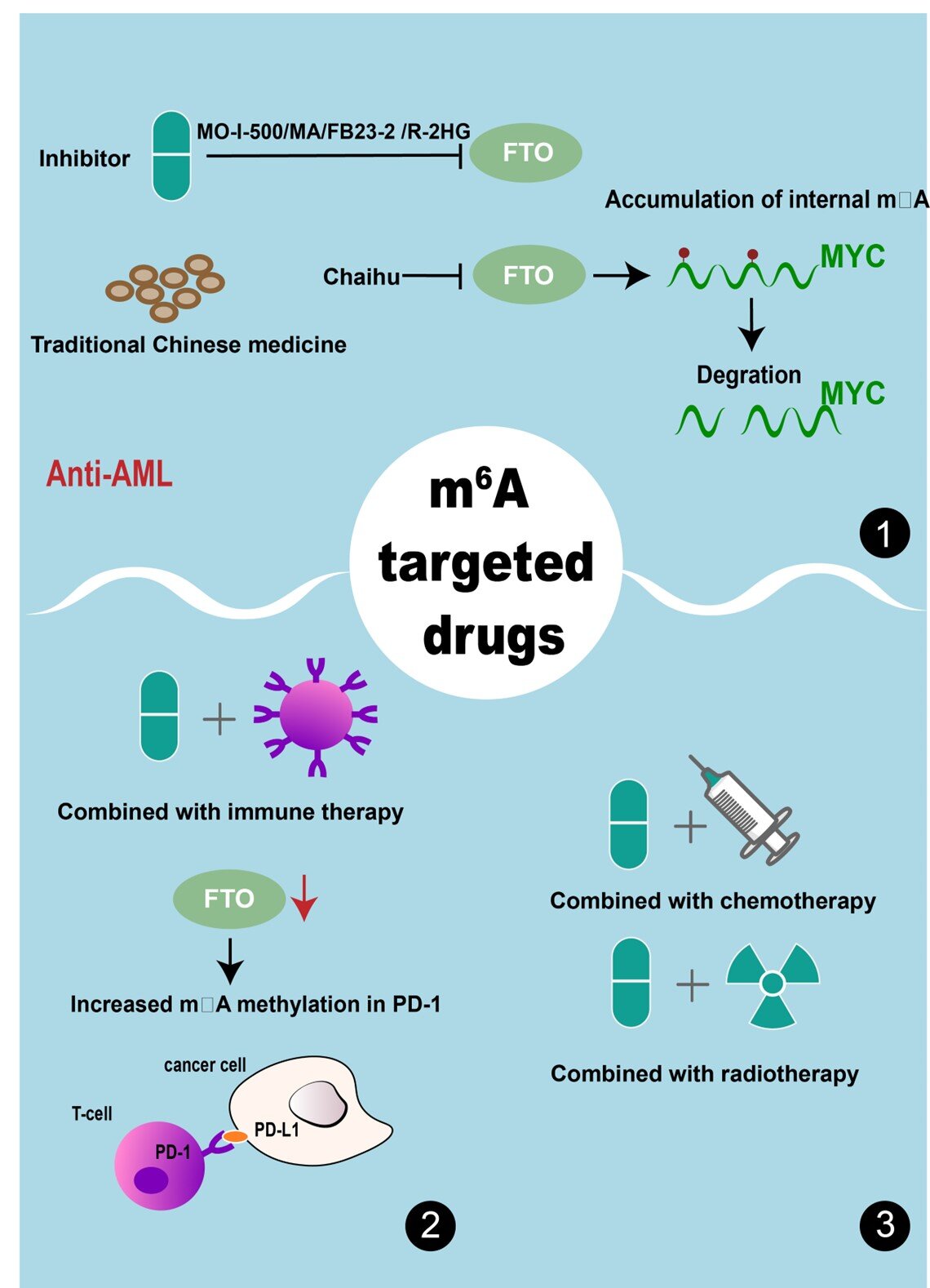
Interestingly, m6A modification regulators exhibit context-specific roles, as they can suppress one cancer type while promoting the growth of another. In AML, regulatory factors in the writers—’methyltransferase-like 3 (METTL3) / methyltransferase-like 14 (METTL14) “, erasers—”Fat mass and obesity-associated (FTO) and AlkB homolog 5,” and reader—”YTH domain family proteins,” have been shown to enhance leukemic cell activity and disrupt normal hematopoietic development. Similarly, the m7G modifying machinery, including the “METTL1-WDR4′ complex, has been associated with cancer initiation in several cancers, including AML.
Furthermore, studies in the field of “epitranscriptomics,” which focuses on post-transcriptionally modified RNA, have revealed the correlation between these aberrant complexes and tumorigenesis, as well as poor prognosis. This correlation makes them promising targets for the development of novel therapeutics.
The review then transitions from discussing the pathogenic roles of RNA-modifying machinery to highlighting the current and impending targeted therapies. Notably, selective inhibitors against FTO and the METTL3-METTL14 complexes have been shown to promote the differentiation of immature cells and reverse the AML phenotype.
Furthermore, Traditional Chinese Medicine compounds, including Huang Qin, curcumin, quercetin, and Rhein, are being increasingly explored for their ability to suppress aberrant m6A RNA methylation and improve therapeutic outcomes following conventional therapies for AML.
Overall, targeting aberrant regulators of post-transcriptional modification can serve as an effective therapeutic strategy in the treatment of AML as well as other cancers. Understanding the fundamental molecular mechanisms underlying normal hematopoiesis and AML pathogenesis can aid the development of novel and effective targeted therapies.
Prof. Wang concludes by saying, “Targeted RNA modification treatment can be put into practice together with traditional anticancer treatments such as chemotherapy, radiotherapy, or immunotherapy to bring about dramatic improvements in cancer treatment within the near future.”

Check out our mRNA service to expedite your vaccine research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
Exploring Tau Protein’s Role in Glaucoma: New Insights and Therapeutic Potential
Glaucoma, a chronic neurodegenerative disorder, leads to irreversible vision loss by damaging retinal ganglion cells (RGCs) and the optic nerve, often associated with increased intraocular pressure (IOP). Despite the benefits of IOP-lowering treatments, the underlying...
FDA-mandated CAR-T monitoring period could be halved, say researchers
In patients with diffuse large B-cell non-Hodgkin lymphoma (DLBCL), the two hallmark post-chimeric antigen receptor (CAR)-T therapy toxicities are extremely rare after two weeks, supporting a shorter, more flexible toxicity monitoring period, according to a study...
Ancestral CRISPR-Cas13 Ribonucleases Discovered: Implications for Genome Editing
In a pioneering study published in *Science*, a team of researchers led by Peter H. Yoon and Jennifer A. Doudna from the University of California, Berkeley, has made a remarkable discovery in the realm of CRISPR technology. The team has identified an ancestral clade...
KBI Biopharma Expands Manufacturing Contract with Global Pharmaceutical Company
KBI Biopharma Inc., a JSR Life Sciences company and global cGMP contract development and manufacturing organization (CDMO), has extended and expanded its manufacturing contract with a leading global pharmaceutical company. Originally initiated in 2020, the renewed...
Related Services

Plasmids GMP Services

AAV GMP Services


