The first DNA editing technologies emerged in the 1990s, but RNA editing technologies didn’t become commercialized until recently, even though evidence of RNA editing occurring in nature was first observed almost 40 years ago (Benne et al. Cell 1986; 46(6): 819–826). An early advance in RNA editing technology occurred in 2012, when scientists found that by linking enzymes to engineered strands of RNA, they could change the sequences of messenger RNA molecules in cells (Stafforst T, Schneider MF. Angew. Chem. Int. Ed. Engl. 2012; 51(44): 11166–11169.).
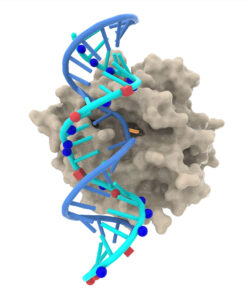
Wave Life Sciences, a clinical-stage RNA medicines company, develops A-to-I RNA base editing oligonucleotides (“AIMers”). AIMers are designed to recruit endogenous adenosine deaminase acting on RNA (ADAR) enzymes to direct highly specific editing of RNA transcripts. Wave asserts that its AIMers are distinct from other ADAR-mediating approaches because they are short, chemically modified, and possess novel features, including proprietary phosphoryl guanidine backbone modifications and chiral control. This image depicts a Wave oligonucleotide/RNA complex engaging with an ADAR enzyme.
Older RNA companies are developing RNA editing programs, and new companies are being established on RNA editing platforms alone. (For its part, Wave Life Sciences recently submitted the first clinical trial application for an RNA editing therapy to enter clinical development.)
Wave Life Sciences
Bolno says that CRISPR should be credited with moving the field beyond silencing and splicing, and toward correction and editing—as well as toward translating genetic insights more broadly. Regardless of the changes wrought by CRISPR, the time, he notes, “was ripe for RNA editing.” There is this notion, he continues, that DNA editing, with its one-and-done treatments, will fix the world. But he wonders whether DNA editing’s promise can distract people from RNA editing’s unique advantages.
Here, we will review some of those advantages, the challenges that lie ahead, and some of the players (both established and brand-new companies) in the hot, new space that is RNA editing. Ultimately, we will see where RNA editing’s momentum is coming from—and where it is leading.
Plusses and minuses
DNA editing and RNA editing share the same end goal: to alter a protein’s structure or quantity. But the two processes take different routes to achieve that goal.
For example, RNA editing is transient, not permanent, because RNA is degraded in cells. Some researchers may consider this a disadvantage, as drugs based on RNA editing will have to be administered more frequently than the one-and-done drugs based on CRISPR. But Bolno says, “I think we’re giving DNA editing a big pass on this because nobody has ever shown a one-and-done result from DNA editing.”
“We also have to remember,” Bolno advises, that “reversibility is not a detrimental feature of making a medicine.” There is a benefit to stopping a drug if necessary. Besides offering a measure of security to patients, reversibility is important within a regulatory and ethical context. The transient nature of RNA edits raises fewer alarm bells than the permanent nature of DNA edits. To support this point, Bolno adds that his company’s RNA editing drug, the first to go into patients, will be tested in healthy volunteers.
Gerard Platenburg, co-founder and CSO of ProQR Therapeutics, sees the durability of the RNA editing oligonucleotide as a strength. He explains that the transient nature of the technology offers flexibility and “opens therapeutic opportunities that were not accessible before.” Oligonucleotides, he continues, can be made to last several months or even longer, allowing infrequent dosing and reducing the burden of treatment for the patient. “Moreover,” he points out, “by making the oligonucleotides less stable, we can tune the effect, making that reversible within a desirable time frame.”
Another potential perk is simplified delivery. RNA editing drugs, made up of modified RNA molecules, may not need to be delivered via lipid nanoparticles or viral vectors. For example, Wave Life Sciences uses short RNA-editing oligonucleotides that are chemically modified with N-acetylgalactosamine (GalNAc), which aids in delivery. The advantage is that GalNAc-conjugated oligonucleotides, unlike the constituents of DNA editing systems, can target the RNA to be taken up by cells. This advantage may end up being one of the biggest differentiators between RNA editing and DNA editing.
Beyond base changes
A discovery made in the late 1980s showed that the enzyme adenosine deaminase acting on RNA (ADAR) was found to unwind double-stranded RNA (dsRNA) in Xenopus. Quickly following that initial discovery, ADAR’s RNA editing ability, through deamination of an adenosine to inosine, was uncovered as a post-transcriptional modification. In the body, ADAR-mediated editing allows the immune system to differentiate between host RNA and foreign RNA that may be pathogenic. The process also aids in the maturation of neurons. The ADAR system, which exists in all cells of the human body and targets adenosine with high specificity, is at the heart of many RNA editing companies’ platforms.
For example, in 2014, ProQR discovered an RNA editing technology that the company calls Axiomer. When ProQR uses Axiomer, the first task is to find RNAs that code for proteins involved in disease. Then the company identifies where an A-to-I edit could be beneficial. However, ADAR cannot recognize the RNA site in single-stranded RNA (ssRNA). Therefore, Axiomer uses short editing oligonucleotides (EONs) that are engineered to bind to the disease-related RNA site, creating a duplex structure that will attract ADAR enzymes, make the A-to-I change, and modify the protein’s function.

To expand the number of diseases that can be altered through A-to-I point mutations, companies are exploring how these mutations can result in a broader array of effects. For example, ProQR is using Axiomer to alter the occurrence of RNA-dependent post-translational modifications such as phosphorylation.
Wave Life Sciences explains that it is also exploring new effects. One mechanism the company favors is the blocking of sites that RNA-binding proteins can exploit to degrade transcripts. According to the company, a treatment based on this mechanism could increase copy numbers and protein expression. The company has (unpublished) data indicating that this mechanism was used across seven targets to resolve protein dysfunctions or deficiencies associated with disease.
Another tool Wave Life Sciences has built is called the Edit-Verse (the editable gene-disease network, which includes coding and noncoding regions of transcripts). The company hopes the Edit-Verse will reveal more of the transcriptome that is eligible for editing. For example, the deep learning model—constructed using large expression quantitative trait loci (eQTL) databases that can predict the impact of editing on gene expression—could identify targets that impact transcriptional regulation because many diseases are associated with reduced protein expression.
Newest entrants
The end of this past summer saw a burst in the number of companies in the RNA editing space. In August, San Francisco–based Amber Bio launched with an oversubscribed $26 million seed financing round. The company says that its RNA editing platform enables multi-kilobase edits—allowing a single drug to treat diseases with high allelic diversity.
Amber’s co-founders are the current CEO, Jacob Borrajo, PhD, who did his graduate work in the laboratory headed by Paul Blainey at MIT and the Broad Institute, and Basem Al-Shayeb, PhD, Amber’s current CTO, who comes to Amber Bio from the laboratory headed by Jennifer Doudna, PhD, at the University of California, Berkeley.
Just a month after Amber Bio’s launch, another company, AIRNA, emerged from stealth with a $30 million initial financing led by ARCH Venture Partners. The founders, Thorsten Stafforst, PhD, professor at the University of Tübingen and Jin Billy Li, PhD, associate professor at Stanford University, have established both a European and American presence for the company with headquarters in Cambridge, MA, and research operations in Tübingen, Germany. The company will focus on ADAR-mediated RNA editing with its RNA editing platform named RESTORE+. AIRNA is advancing the development of a candidate to treat the inherited genetic disease alpha-1 antitrypsin deficiency (AATD). The company indicates that its aim is to target multiple prevalent diseases with high unmet need.
A review written by Stafforst and his graduate student Laura Pfeiffer was published in September in Nature Biotechnology. “We expect the field to realize the first RNA base-editing drug soon, likely on a well-defined genetic disease,” they wrote. “However, the long-term challenge will be to carve out the sweet spot of the technology where its unique ability is exploited to modulate signaling cues, metabolism, or other clinically relevant processes in a safe and doseable manner.”
In other news, the RNA editing company Korro Bio and regenerative medicine company Frequency Therapeutics, announced that they have entered into a definitive merger agreement to combine the companies in an all-stock transaction. Korro’s RNA editing platform—Oligonucleotide Promoted Editing of RNA (OPERA)—stems from research in the laboratory of Josh Rosenthal, PhD, a neurobiologist at the Marine Biological Laboratory in Woods Hole, MA. His research into marine organisms, and their adaptation to physical environments, led him to focus on RNA editing.
Korro’s lead program focuses on a disease-modifying therapy for patients with AATD. The company’s preclinical data showed an increase of normal alpha-1 antitrypsin protein to 85% of total protein in circulation.
All these companies, and multiple others working in RNA editing, exist in a newly defined field. Indeed, RNA editing companies, Bolno observes, are “kind of side invitees” to meetings that are focused on topics such as cell and gene therapy, oligonucleotides, and genome editing. But one thing remains consistent: There are many ways to develop genetic medicines. And, as Bolno adds, “A whole bunch of people have a whole bunch of really innovative ways to interrogate it.”
To the clinic … and beyond
In September, Wave Life Sciences announced that it had submitted a clinical trial application for the first RNA editing clinical candidate, WVE-006. The company indicated that it is “on track for dosing for in Q4.” WVE-006 is designed to restore production and circulation of functional, wild-type alpha-1 antitrypsin protein, and to reduce levels of mutant Z-alpha-1 antitrypsin protein, thereby addressing alpha-1 antitrypsin deficiency-related lung disease, liver disease, or both.
Bolno tells GEN that it’s thrilling to be moving into the clinic. And he is looking forward to 2024, when his company will have the first data on editing—data that will unlock the proof of mechanism. But what is really exciting, he asserts, is that we are seeing “the realization of translation of genetics.”
https://www.genengnews.com/topics/genome-editing/rna-editing-is-having-a-moment/
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
Exploring Tau Protein’s Role in Glaucoma: New Insights and Therapeutic Potential
Glaucoma, a chronic neurodegenerative disorder, leads to irreversible vision loss by damaging retinal ganglion cells (RGCs) and the optic nerve, often associated with increased intraocular pressure (IOP). Despite the benefits of IOP-lowering treatments, the underlying...
FDA-mandated CAR-T monitoring period could be halved, say researchers
In patients with diffuse large B-cell non-Hodgkin lymphoma (DLBCL), the two hallmark post-chimeric antigen receptor (CAR)-T therapy toxicities are extremely rare after two weeks, supporting a shorter, more flexible toxicity monitoring period, according to a study...
Ancestral CRISPR-Cas13 Ribonucleases Discovered: Implications for Genome Editing
In a pioneering study published in *Science*, a team of researchers led by Peter H. Yoon and Jennifer A. Doudna from the University of California, Berkeley, has made a remarkable discovery in the realm of CRISPR technology. The team has identified an ancestral clade...
KBI Biopharma Expands Manufacturing Contract with Global Pharmaceutical Company
KBI Biopharma Inc., a JSR Life Sciences company and global cGMP contract development and manufacturing organization (CDMO), has extended and expanded its manufacturing contract with a leading global pharmaceutical company. Originally initiated in 2020, the renewed...
Related Services

