PackGene has pioneered innovative approaches to meet the escalating demand for high-quality adeno-associated virus (AAV) vectors across the dynamic landscape of gene therapy. For example, PackGene achieves remarkable enhancements in AAV packaging efficiency with a 3 to 8-fold increase in AAV production yield across various serotypes through their proprietary π-Alpha 293 AAV High-Yield Platform. The process began with the development of the PCS3.0 suspension cell line, which exhibits substantial improvements in production yield compared to its predecessors. PackGene meticulously engineered the PCS3.0 cell line using cutting-edge technologies to optimize for unparalleled productivity, stability, and quality. This PCS3.0 suspension cell line was then coupled with comprehensive process optimization and the introduction of a novel dual-plasmid system to further enhance AAV productivity and quality while minimizing the risk of contamination. Overall, PackGene’s commitment to innovation has revolutionized AAV production by driving transformative advancements in gene therapy.
Introduction
PackGene has pioneered innovative approaches to meet the escalating demand for high-quality adeno-associated virus (AAV) vectors across the dynamic landscape of gene therapy. For example, PackGene achieves remarkable enhancements in AAV packaging efficiency with a 3 to 8-fold increase in AAV production yield across various serotypes through their proprietary π-Alpha 293 AAV High-Yield Platform. The process began with the development of the PCS3.0 suspension cell line, which exhibits substantial improvements in production yield compared to its predecessors. PackGene meticulously engineered the PCS3.0 cell line using cutting-edge technologies to optimize for unparalleled productivity, stability, and quality. This PCS3.0 suspension cell line was then coupled with comprehensive process optimization and the introduction of a novel dual-plasmid system to further enhance AAV productivity and quality while minimizing the risk of contamination. Overall, PackGene’s commitment to innovation has revolutionized AAV production by driving transformative advancements in gene therapy.
π-Alpha 293 AAV High-Yield Platform
PackGene’s proprietary π-Alpha 293 AAV High-yield Platform uses a combination of technological advancements to increase AAV production yield and quality. First, the platform incorporates PackGene’s PCS3.0 suspension cell line and uniquely designed RC plasmid in the triple-plasmid transfection system to increase production yield 3 to 8 times for various AAV serotypes. These technologies are coupled with both in-process upstream and downstream QbD optimizations to increase total AAV yield up to 10-fold. A single batch of AAV production delivers up to 1E+17vg virus particles, which is enough to meet the needs of most clinical and commercial level of AAV production requirements.
Advantages of the Platform
- Productivity: Proprietary technologies elevate AAV yield in HEK293 serum-free cell suspension systems by over 10-fold.
- Quality: Unique process development procedures significantly diminish key impurities such as Host Cell DNA (HCD) and endotoxin.
- Viability: Key technological innovations reduce the rate of empty capsids and enhance infection titers.
High-Yield AAV Production Cell Line: From PCS 2.0 to PCS 3.0
PackGene’s pursuit of high-yield AAV production began with the development of an optimized single clonal suspension cell line. Derived from the ATCC HEK293 cells (Grieger JC et al.), PackGene’s PCS 3.0 monoclonal cell line yields impressive results. With Production yield enhancements of PCA (adherent cell line) up to 7x titer and PCS 2.0 (suspension cell line) up to 4x titer.
PackGene recognized the crucial role of cell substrates in AAV manufacturing, and thus began the process of optimizing AAV production by generating a cell line that is specifically engineered for high efficiency AAV production. To this end, PackGene designed a stage-wise cell engineering protocol to identify cells that are optimized for AAV production. The PCS2.0 monoclonal cell line was chosen as the initial cell line due to its suspension growth capability, high efficiency in producing AAV, and widespread use in AAV production. Multiple highly uniform monoclonal cells lines were generated from PCS2.0 cultures using Dispencell in-situ cell dispensing and a CloneSelect Imager for clonality verification. Each generated cell line was then screened for AAV production yield and Population Doubling Time (PDT) in order to identify the most efficient clone (Figure 1). The most efficient clone was then selected and further refined through gradient-decreasing serum cultures and two rounds of single-cell sorting (Figure 2). The resultant PCS3.0 monoclonal cell line is a robust suspension cell line with unparalleled AAV productivity, stability, and quality.
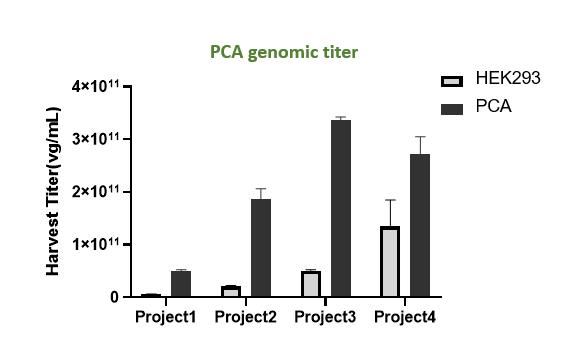
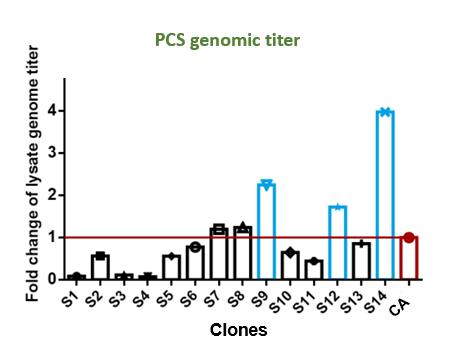
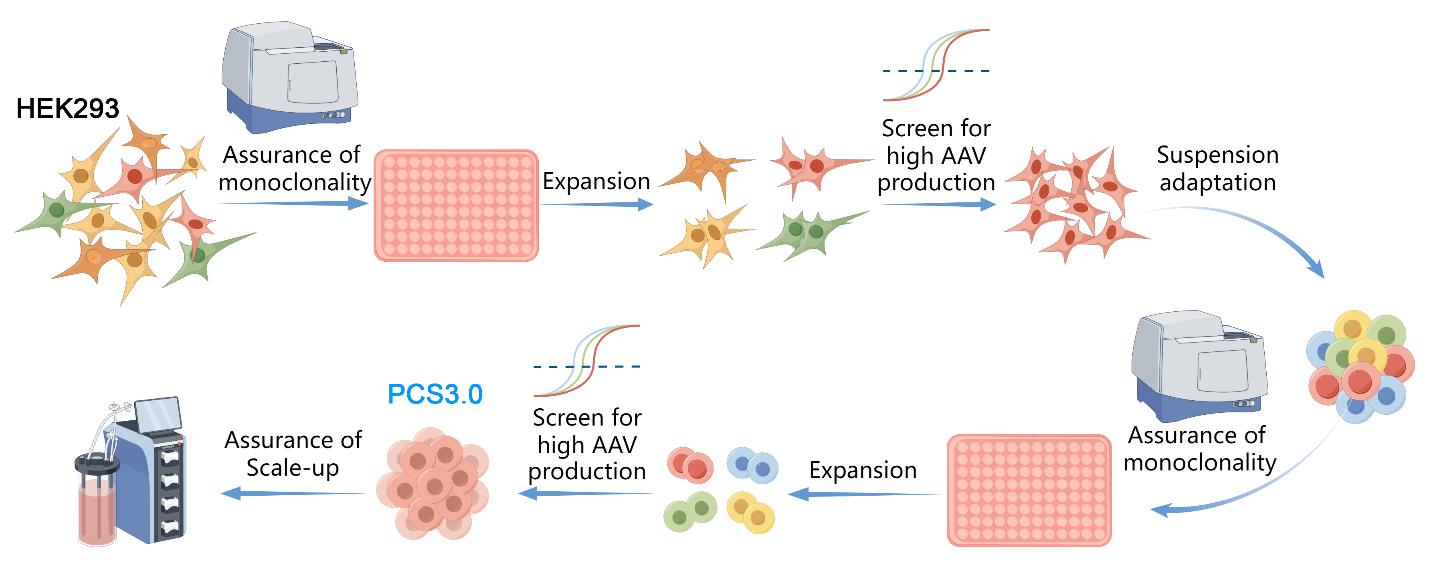
Figure 2. Overview of PCS3.0 cell line development
The results of these studies indicated comparable AAV yields between VPC2.0 and PCS3.0 (Table 1, Figure 3A). However, PCS3.0 exhibited several advantages over VPC2.0. Notably, PCS3.0 demonstrated significantly lower levels of residual host cell DNA (HCD) and residual plasmid DNA (pDNA) compared to VPC2.0. Encapsulated pDNA levels were found to be reduced up to threefold ( 50~60 ng/E+12vg). Additionally, under the 3L growth condition, PackGene observed that PCS3.0 displayed reduced glucose consumption and slower accumulation of metabolic waste (lactate).
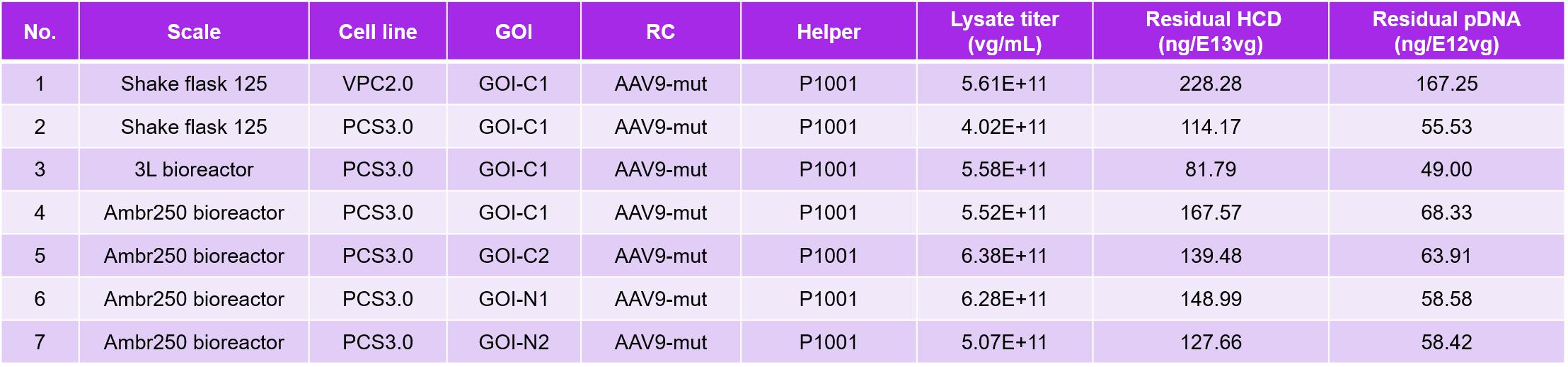
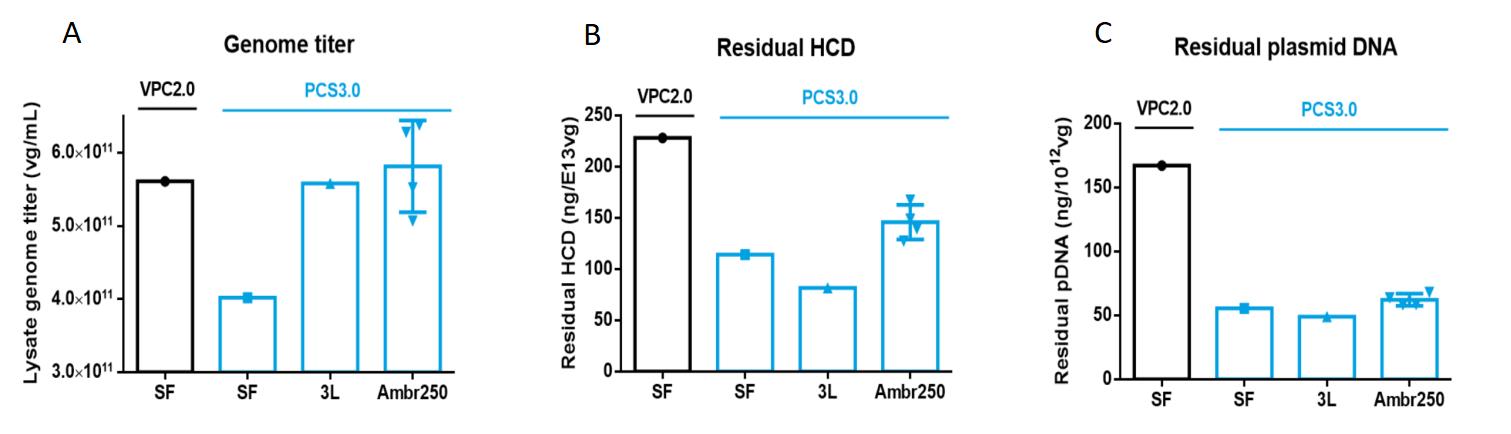
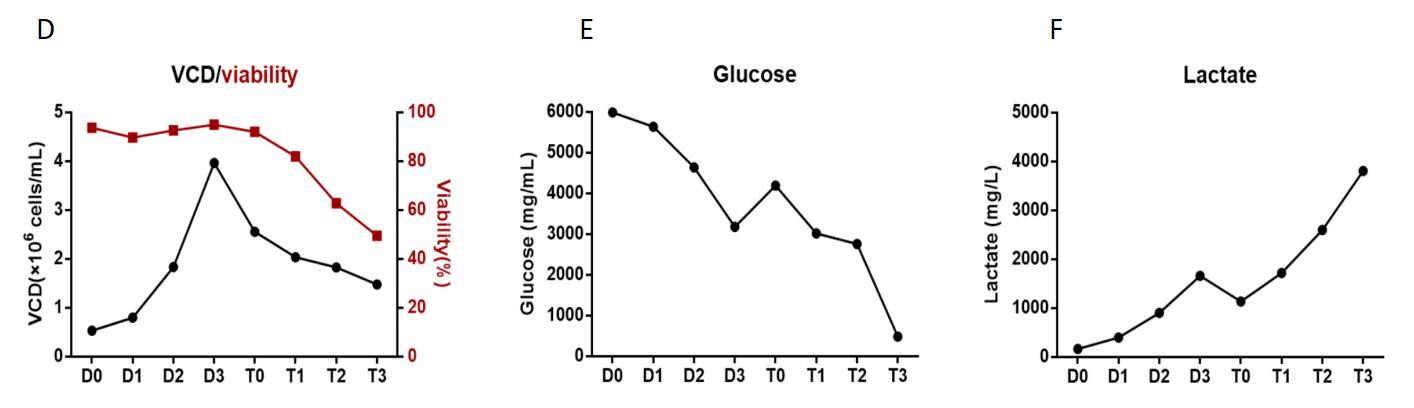
Figure 3. PCS3.0 cell line in a ssAAV9 gene therapy project
In addition to selecting the idea cell line, PackGene recognized that optimization of Critical Process Parameters (CPP) can substantially impact AAV production efficiency and quality. PackGene performed parametric optimization analysis across (1) transfections conditions (2) engineering parameters and (3) lysis and harvest conditions (Table 2). Combined these CPP optimizations ensure maximal AAV production efficiency.
| Critical Process Parameters | ||
| Transfection Conditions | Engineering Parameters | Harvest conditions |
| Cell density | Stir speed | Stir speed |
| Total plasmid amount | pH | Cell density |
| Plasmid ratio | Dissolved oxygen (dO2) | Time |
| DNA/PEI ratio | Temperature | Temperature |
Table 2. Optimized Critical Process Parameters
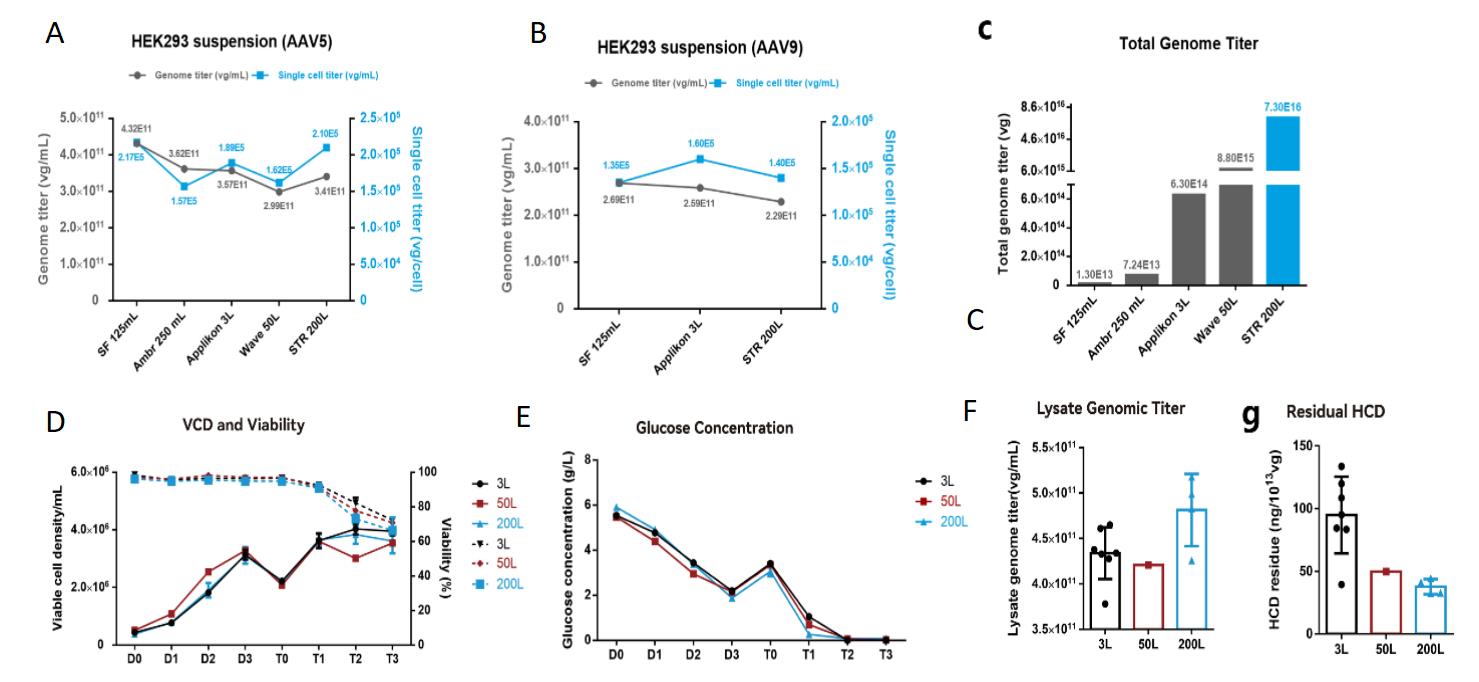
With Critical Process Parameters optimized, PackGene’s suspension production process demonstrates robust amplification across varying scales with an overall recovery rate that exceeds 30%. For example, scaling up to 200L AAV production volume with stable genomic titer and residual hcDNA resulted in 1.0E17 vg at harvest. Critically, Key Product Quality Attributes, including significant full capsid enrichment, were maintained in the 76-92% range during scale-up. Thus, up-scaling production using PackGene’s optimized 𝝅-Alpha 293 High-yield AAV Production Platform meet even stringent GMP production requirements.
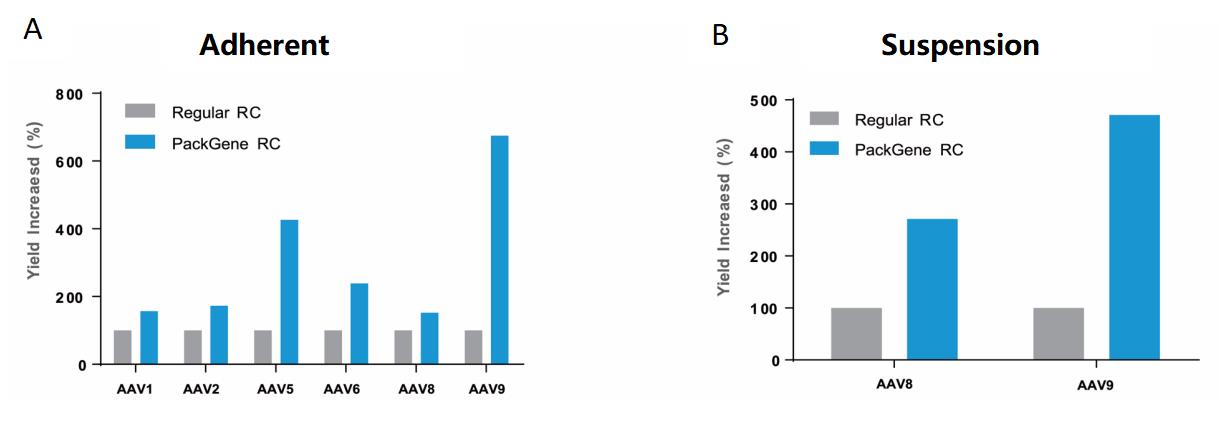
PackGene designed and validated a proprietary non-coding regulatory element with the goal of enhancing AAV yield. Incorporation of this regulatory element into a novel Rep-Cap plasmid enhanced AAV yield in both adherent and suspension cell lines by 3-8 times across various serotypes (Figure 5). In addition, PackGene found that AAV production using their novel RC Plasmid reduced the observed plasmid DNA encapsulation ratio from 3% to between 0.1% and 0.2% (Data not shown).

The traditional triple-plasmid transfection system is a cornerstone in industrial AAV manufacturing due to its scalability. An alternative dual-plasmid system has emerged as a cost-saving and simplified procedure for AAV manufacturing (Matsushita T et al., Grimm D et al., Tang Q et al.). While there has been some adoption of the dual-plasmid approach, persistent challenges including low productivity, quality issues, and the risk of generating replication-competent AAV (rcAAV) necessitated further innovation.
PackGene sought to design and validate a dual-plasmid AAV production system that mitigates the challenges that are common to other dual-plasmid AAV production systems. Toward this end PackGene designed a novel TP-plasmid that integrates replication-competent (RC) genes, and helper functions into a single plasmid. Additional experiments were performed to integrate this novel TP-plasmid with PackGene’s 𝝅-Alpha 293 High-yield AAV Production Platform by parametrically testing transfection reagents. The resultant TP011-dual plasmid AAV production system was tested against traditional triple plasmid AAV production methods and was found to show increases yield and reductions in host cell DNA (HCD) residue. This result was consistent across production scales, which positions PackGene’s TP011 dual-plasmid system as a superior AAV production platform.
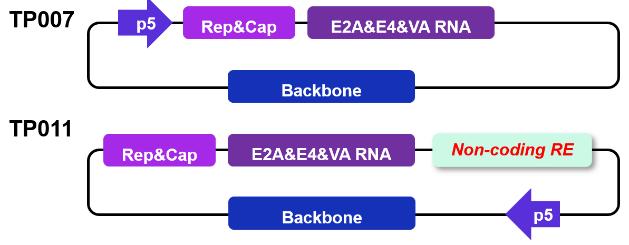
Non-rcAAV design of packing plasmids for dual-plasmid system.
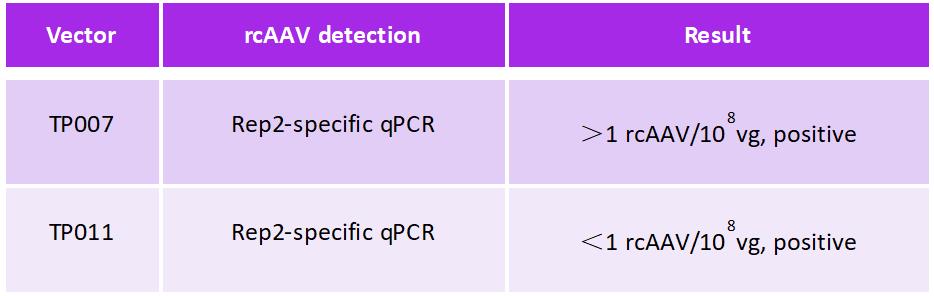
Triple-plasmid AAV production systems require three separate plasmids, one plasmid that holds the gene of interest (GOI), a second plasmid that contains replication-competent (RC) genes, and a third plasmid that contains helper genes required for AAV packaging. PackGene’s dual-plasmid system requires only two plasmids, one plasmid that holds the gene of interest (GOI) and a second TP plasmid that holds both replication-competent (RC) genes and helper genes. PackGene’s initial attempts to design a TP plasmid resulted in the TP007 plasmid. AAV generation with the TP007 plasmid was successful, however, rcAAV detection by Rep-2 specific qPRC showed less than ideal rcAAV generation rates (Figure 6). To mitigate this issue, TP007 was modified to include a proprietary non-coding regulatory element that was designed by PackGene (Figure 6A). Inclusion of this regulatory element in the new TP011 vector showed a substantial reduction in rcAAV generation (Figure 6B). AAV generation with a dual-plasmid system that shows low rcAAV generation levels marks a significant leap forward in AAV production technology.
PackGene paired their innovative dual-plasmid system with their 𝝅-Alpha 293 High-yield AAV Production Platform Process Optimization to maximizes AAV yield and to ensures consistent and high-quality vector production. This alone enhances the efficacy and reliability of PackGene’s AAV production platform. Nevertheless, PackGene went several steps further to fully integrate these two innovations. Transfection reagents (TR) were screened for use within the dual-plasmid system (Figure 7A) and identified a small molecule compound that significantly enhanced AAV9 productivity on different TRs (Figure 7B).
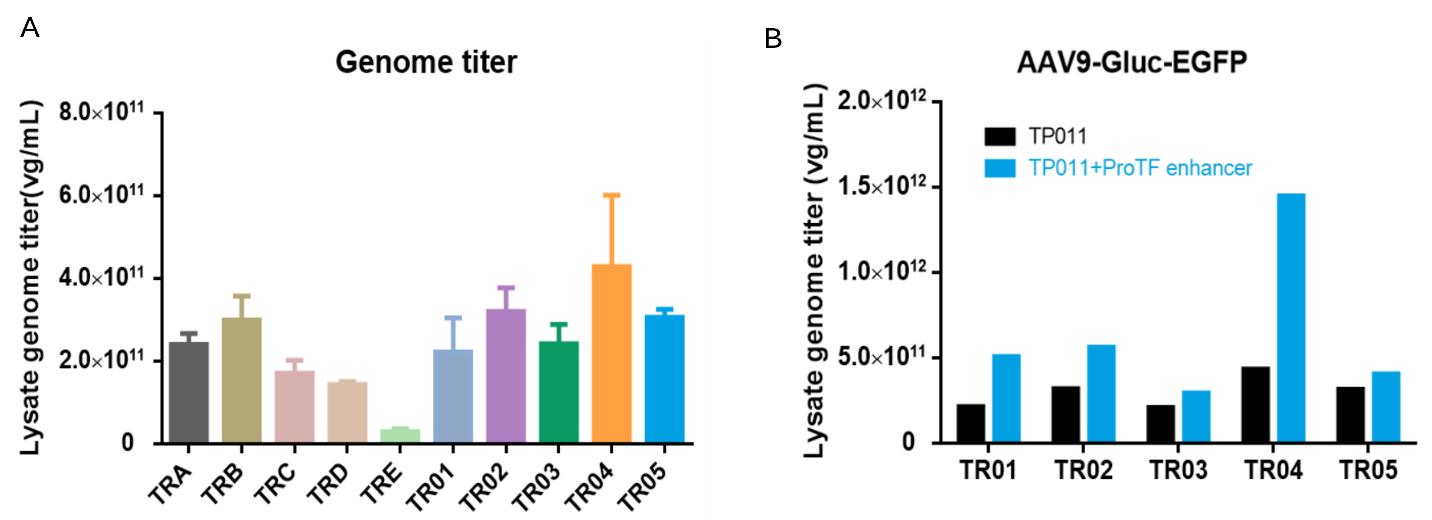
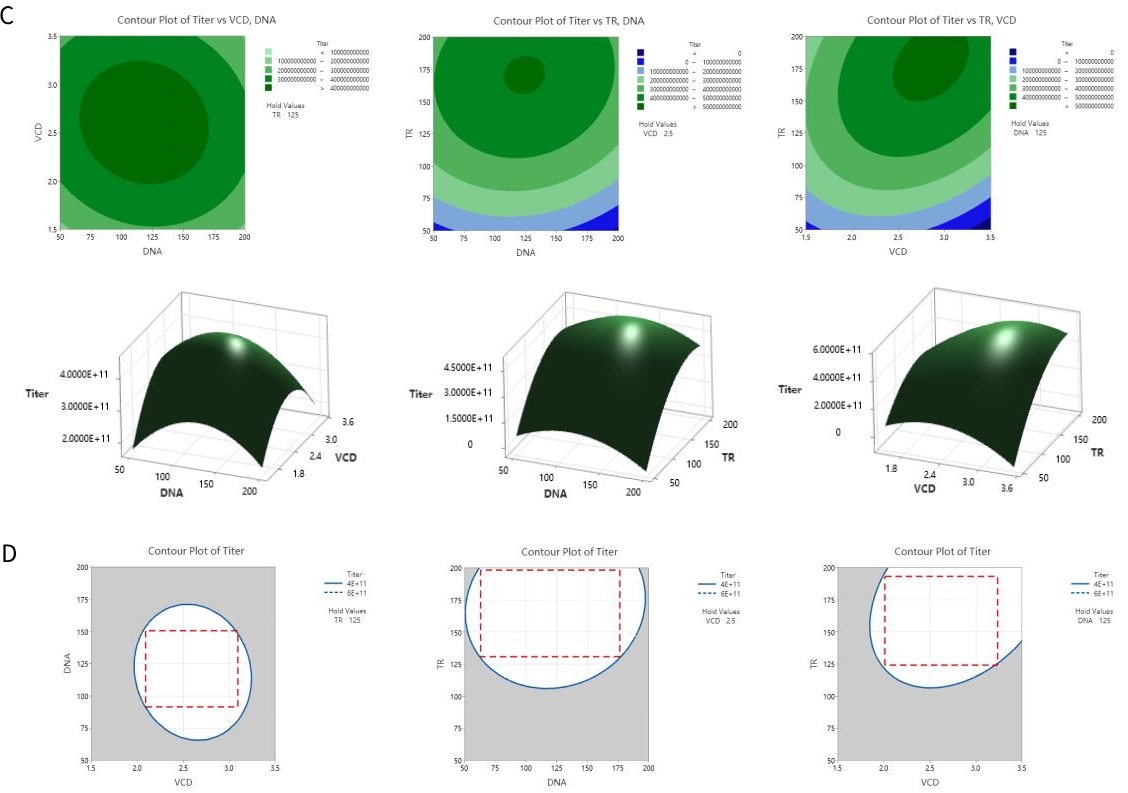
Figure 7. DoE optimization enabling high productivity
A-B. Different transfection reagent and effect of a small molecule on AAV9 productivity. C. Contour plots of a Box-Behnken design (BBD) DoE experiment on TP011 dual-plasmid system to optimize transfection viable cell density (VCD), plasmid DNA and TR amount. For AAV2-EA0218K, a platform GOI, DoE optimized dual-plasmid transfection process can harvest >5.0×1011 vg/mL AAV lysate; D. CPP designed space of VCD/DNA/TR can outcome a stable AAV2-EA0218K yield between 4.0~6.0 ×1011 vg/mL.
TP011 dual-plasmid system with higher yield and lower HCD residue.
PackGene tested the efficiency and quality of their TP011 dual-plasmid system by comparing AAV9 production titer and residual host cell DNA (HCD) residue to AAV generated using traditional triple-plasmid production methods. They found that AAV9 yield was more than 3x higher when using the TP011 dual-plasmid system when compared to the traditional triple plasmid methods (Table 3; Figure 8A). In addition, they found that residual HCD was more than 3x lower in TP011 dual-plasmid system samples relative to triple-plasmid methods while residual pDNA was comparable (Table 3; Figure 8B, C).
PackGene also sought to determine if improvements in AAV production titer were stable regardless of the GOI plasmid used for production. To test this they compared TP011 dual-plasmid system AAV9 production titer to titers of AAV generated by triple-plasmid production methods using 4 distinct GOI plasmids. For two of the GOI plasmids it was found that AAV9 generated by the TP011 dual-plasmid system showed elevated titer relative to triple-plasmid production methods. AAV9 titers were comparable between the two methods for the remaining two GOI plasmids (Figure 8D). This data serves as evidence that PackGene’s TP011 dual-plasmid system represents an advancement in AAV production technology by showing superior yield with reduced HCD residue. Thus, the TP011 dual-plasmid system streamlines the production process, resulting in heightened yield and purity.
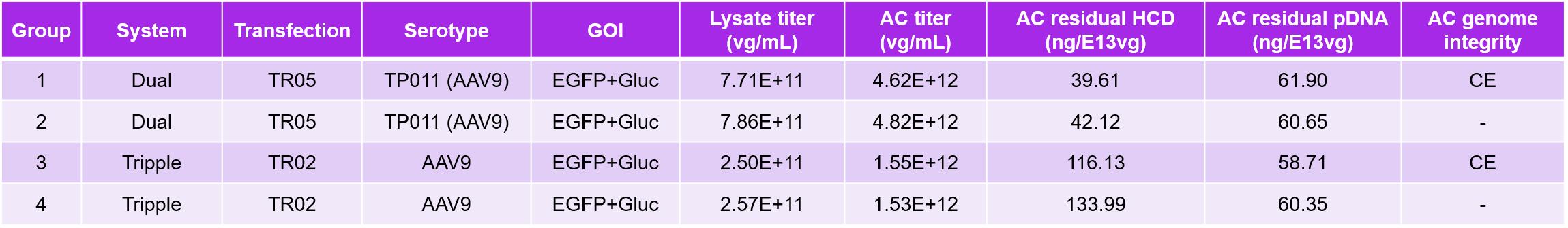
Table 3. TP011 dual-plasmid system and traditional triple-plasmid method comparison parameters
Application of dual plasmid system in a therapeutic case study
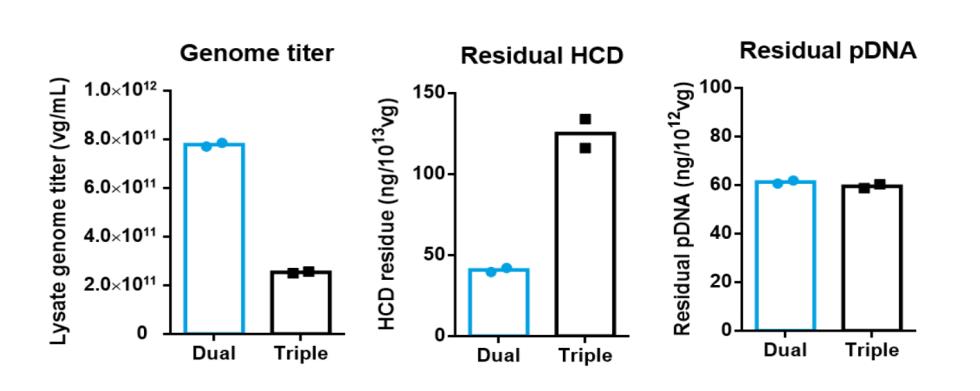
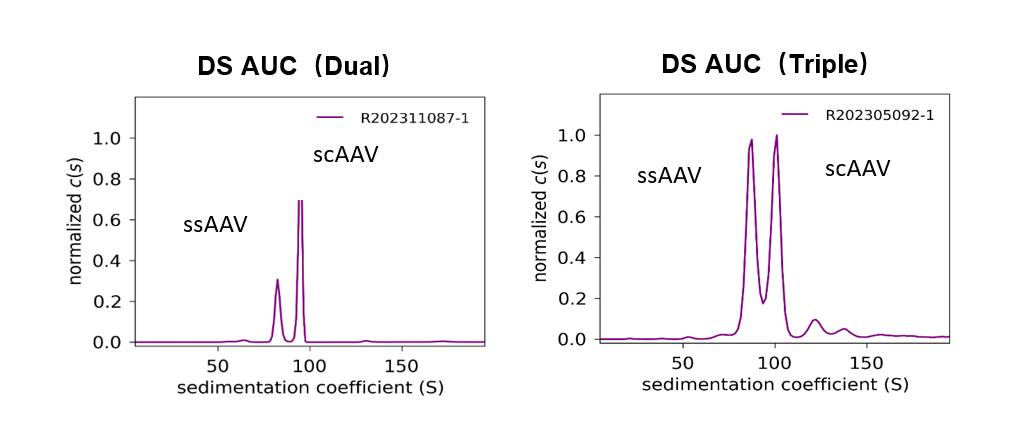
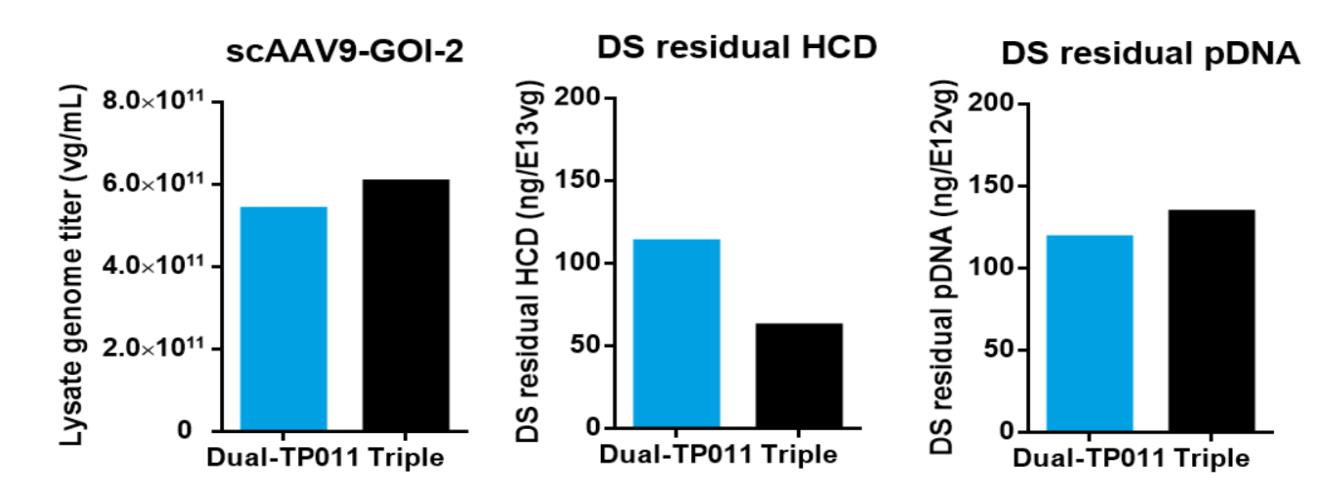
PackGene has used their 𝝅-Alpha 293 High-yield AAV Production Platform with their TP011 dual-plasmid system to generate AAV for numerous labs across the world. Here we present one case study in which PackGene generated scAAV9 for a real-world application, with a comparison to the same AAV9 generated by traditional triple-plasmid methods (Table 5). PackGene found similar scAAV9 AAV lysate yield, residual HCD and pDNA impurities when their TP011 dual-plasmid system with the traditional triple-plasmid generation method (Table 5; Figure 9). Additionally, there was no significant difference in AAV empty capsid ratio the two approaches, and Analytical Ultracentrifuge (AUC) shows a relative smaller ssAAV peak and larger scAAV peak compared to triple-plasmid system, with empty AAV peak as low as 1.50%. The higher scAAV ratio indicates the therapeutic gene can express directly and more rapidly, with higher expression levels after entering the cell.

PackGene’s unwavering dedication to innovation and excellence has propelled the advancement of high-yield AAV production platforms, ushering in a new era in gene therapy. The development of the PCS3.0 cell line and our novel dual-plasmid system epitomize PackGene’s commitment to pushing the boundaries of AAV manufacturing processes, equipping researchers and clinicians with potent tools to expedite the development of groundbreaking therapeutics.
As the field of gene therapy continues to evolve, PackGene remains at the forefront, driving innovation and shaping the future of AAV production. With an unwavering commitment to excellence, PackGene is spearheading transformative advancements in gene therapy, paving the way for revolutionary medical breakthroughs.
For deeper insights into PackGene’s cutting-edge AAV production technologies, we encourage you to explore our video and visit our website. Immerse yourself in the future of plasmid design and AAV production with PackGene, where innovation knows no bounds.
- Grieger JC, Soltys SM, Samulski RJ. Production of Recombinant Adeno-associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol Ther. 2016 Feb;24(2):287-297. doi: 10.1038/mt.2015.187. Epub 2015 Oct 6. PMID: 26437810; PMCID: PMC4817810.
- Grimm D. Kay M.A. Kleinschmidt J.A. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol. Ther. 2003; 7: 839-850
- Matsushita T. Elliger S. Elliger C. Podsakoff G. Villarreal L. Kurtzman G.J. Iwaki Y. Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998; 5: 938-945
- Tang Q. Keeler A.M. Zhang S. Su Q. Lyu Z. Cheng Y. Gao G. Terence T.F. Two-plasmid packaging system for recombinant adeno-associated virus. Biores Open Access. 2020; 9: 219-228
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
More Articles
The principles and advantages of constructing self-complementary double-stranded AAV (scAAV)
Recombinant adeno-associated virus (rAAV) serves as a gene delivery vector in vivo, where the necessary condition for transcription to proceed is the conversion of the genome sequence from single-stranded AAV (ssAAV) to double-stranded AAV. When it comes to the...
AAV Serotype Screening System
Recombinant adeno-associated viruses (rAAV) are the most commonly used delivery vectors in gene therapy. However, naturally occurring AAVs with organ-targeting properties are very limited. In order to achieve more precise treatment goals, scientists have continuously...
Adeno-associated virus (AAV) Packaging: A Comprehensive Exploration of its Packaging, Mechanisms, and Diverse Applications
Adeno-associated virus (AAV) is a member of the Parvoviridae family, a type of non-enveloped icosahedral virus with a diameter of approximately 20-26 nm and containing around 4.7 kb of linear single-stranded DNA as its genome. Recombinant adeno-associated virus (rAAV)...
Related Services
AAV CDMO Services
READ MORE
AAV Packaging (Research Grade)
READ MORE
AAV Packaging (NHP Grade)
READ MORE

