Hepatitis refers to inflammation of the liver, and is usually caused by viral infections, toxins (including alcohol), medications, autoimmune conditions or inherited conditions. Hepatitis is unusual in children and when it does occur, cases are generally isolated.
The children had presented to hospital mostly with a history of gastrointestinal illness weeks earlier, with jaundice, and in some cases with severe liver failure. PHS initiated an urgent investigation of these cases which were noted to affect children of nursery or primary school age, most of whom had been otherwise fit and healthy.
PHS officials informed their counterparts in England at the UK Health Security Agency (UKHSA), who noted that they too were seeing an increase in such cases.
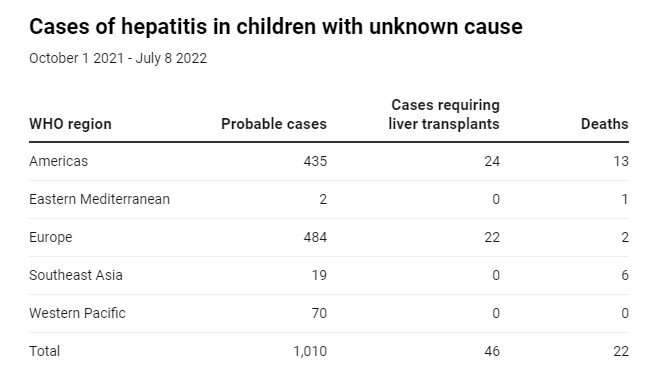
The UK public health agencies alerted the World Health Organization (WHO), and by the summer of 2022, more than 1,000 children around the world were reported to have been affected—the majority in Europe and the Americas. Around half of the European cases occurred in the UK.
The consequences for some children were severe: 22 died and 46 required a liver transplant.
We still don’t fully understand what caused this outbreak, which has now subsided. But three new studies published in Nature offer important clues.
Children who had been diagnosed with hepatitis were invited to give blood samples with consent from their parents or guardians. The scientists looked for viruses that commonly cause hepatitis in children, as well as for rarer conditions and toxins.
These tests didn’t reveal a clear cause, although it was noted that many of the children had tested positive for the human adenovirus F41 (HAdV F41)—a common cause of gastrointestinal infection in children, but not hepatitis.
Both laboratories identified that most of the children with hepatitis had evidence of a virus called adeno-associated virus 2 (AAV2) in their bloodstream, and also in the liver samples from those children who had required a liver biopsy. For example, we detected AAV2 in 26 out of 32 cases of hepatitis, compared with only five out of 74 healthy children we studied as a comparison group.
HAdV F41 was also detected in several (but not all) cases, in blood, liver and gastrointestinal samples.
AAV2 belongs to a group of viruses called Dependoparvovirus. It is known to replicate in the liver, but to do so it usually requires a “helper” virus such as an adenovirus like HAdV F41 (hence its name), or other viruses such as herpesviruses. AAV2 has not previously been found to cause illness.
In our research, we were able to see AAV2 in diseased liver cells using a technique called in situ hybridisation. We also measured the immune response to AAV2 in affected children, and found most of these children had evidence of IgM antibodies in their bloodstream, which indicates a recent infection.
Our results suggest that AAV2 may have been the cause of this outbreak of hepatitis in children. However, as it co-occurs with adenovirus infection, it could be that HAdV F41 is responsible as well. Further studies will be required to confirm which virus is the most likely cause.
Working with experts around Scotland, we also found that most of affected children had an underlying genetic susceptibility. Some 93% of the children with hepatitis carried a gene that’s linked with the immune response directed by T cells, compared with 16% in the control group. The presence of this gene means that hepatitis in these cases is likely to have been related to an over-reactive immune response.
The UK and US authors concluded that changes in the circulation of viruses after COVID lockdowns may have increased the chances of children being exposed for the first time to multiple viruses. Our paper shows that a wave of adenovirus infection immediately preceded the pediatric hepatitis cases in Scotland, but samples were not available to look at the circulation of AAV2.
Interestingly, a recent publication from Ireland confirmed that both adenovirus and AAV2 viruses peaked in sewage samples taken from the Ringsend wastewater treatment plant in Dublin immediately before a similar outbreak of pediatric hepatitis in Ireland.
While these findings suggest strongly that AAV2, HAdV F41 and the immune response to one or both of these viruses underlie the cases of hepatitis seen around the world, larger studies are needed to confirm the findings. We also need to develop and test new treatments for affected children in case a new outbreak occurs.
PackGene is a world-leading AAV vector CRO and CDMO company. We provides economical, reliable, and scalable plasmid DNA and AAV viral vector production for early-stage drug discovery, preclinical development and clinical trials for Cell and Gene Therapy (CGT). If you want more information about our AAV services, please contact us.
Related News
Exploring Tau Protein’s Role in Glaucoma: New Insights and Therapeutic Potential
Glaucoma, a chronic neurodegenerative disorder, leads to irreversible vision loss by damaging retinal ganglion cells (RGCs) and the optic nerve, often associated with increased intraocular pressure (IOP). Despite the benefits of IOP-lowering treatments, the underlying...
FDA-mandated CAR-T monitoring period could be halved, say researchers
In patients with diffuse large B-cell non-Hodgkin lymphoma (DLBCL), the two hallmark post-chimeric antigen receptor (CAR)-T therapy toxicities are extremely rare after two weeks, supporting a shorter, more flexible toxicity monitoring period, according to a study...
Ancestral CRISPR-Cas13 Ribonucleases Discovered: Implications for Genome Editing
In a pioneering study published in *Science*, a team of researchers led by Peter H. Yoon and Jennifer A. Doudna from the University of California, Berkeley, has made a remarkable discovery in the realm of CRISPR technology. The team has identified an ancestral clade...
KBI Biopharma Expands Manufacturing Contract with Global Pharmaceutical Company
KBI Biopharma Inc., a JSR Life Sciences company and global cGMP contract development and manufacturing organization (CDMO), has extended and expanded its manufacturing contract with a leading global pharmaceutical company. Originally initiated in 2020, the renewed...
Related Services

AAV cGMP Manufacturing
Comply with GMPs, all applicable regulatory and the standards for IIT & IND applications.
READ MORE

AAV Packaging Services
Ranging from pilot to industrial-scale AAV packaging for both in vitro and in vivo studies.
READ MORE

AAV Capsid Engineering
Proven technology paving your path to effective therapies for cancer or genetic disorder
READ MORE

