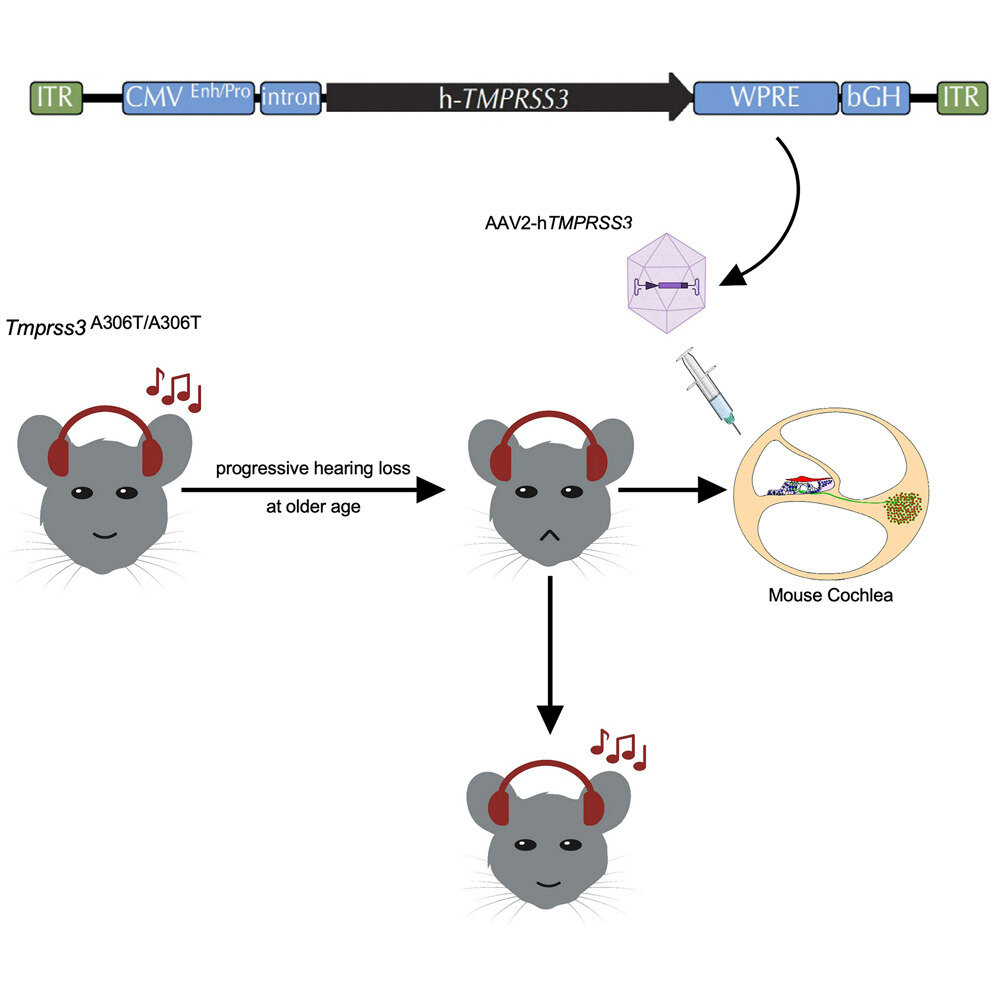
Graphical abstract. Credit: Molecular Therapy (2023). DOI: 10.1016/j.ymthe.2023.05.005
While hearing aids and cochlear implants offer limited relief, no available treatment can reverse or prevent this group of genetic conditions, prompting scientists to evaluate gene therapies for alternative solutions.
One of the most promising tools used in these therapies—adeno associated virus (AAV) vectors—has galvanized the hearing-loss community in recent years.
Despite having already rescued hearing in neonatal animals with genetic defects, the vectors have yet to demonstrate this ability in fully mature or aged animal models. Since humans are born with fully developed ears, this proof-of-concept is necessary before testing the intervention in humans with genetic hearing loss.
A team of researchers from Mass Eye and Ear, a member of Mass General Brigham, recently became the first to successfully demonstrate AAV vector efficacy in aged animal models when they developed a mature mouse model with a mutation equivalent to a defective TMPRSS3 human gene, which typically results in progressive hearing loss.
As reported in Molecular Therapy, researchers observed robust hearing rescue in the aged mice upon injecting the animals with an AAV carrying a healthy human TMPRSS3 gene.
“Our findings suggest that a virally mediated gene therapy, either by itself or in combination with a cochlear implant, could potentially treat genetic hearing loss,” said corresponding author Zheng Yi Chen, D.Phil., an investigator in the Eaton-Peabody Laboratories at Mass Eye and Ear.
“This was also the first study that has rescued hearing in aging mice, which points to the feasibility of treating DFNB8 patients with DFNB8 even at an advanced age. The study also establishes the feasibility of other gene therapies in the aged population.”
More information: Wan Du et al, Rescue of auditory function by a single administration of AAV-TMPRSS3 gene therapy in aged mice of human recessive deafness DFNB8, Molecular Therapy (2023). DOI: 10.1016/j.ymthe.2023.05.005
Journal information: Molecular Therapy
PackGene is a CRO & CDMO technology company that specializes in packaging recombinant adeno-associated virus (rAAV) vectors. Since its establishment in 2014, PackGene has been a leader in the AAV vector CRO service field, providing tens of thousands of custom batches of AAV samples to customers in over 20 countries. PackGene offers a one-stop CMC solution for the early development, pre-clinical development, clinical trials, and drug approval of rAAV vector drugs for cell and gene therapy (CGT) companies that is fast, cost-effective, high-quality, and scalable. Additionally, the company provides compliant services for the GMP-scale production of AAVs and plasmids for pharmaceutical companies, utilizing five technology platforms, including the π-Alpha™ 293 cell AAV high-yield platform and the π-Omega™ plasmid high-yield platform. PackGene’s mission is to make gene therapy affordable and accelerate the launch of innovative gene drugs. The company aims to simplify the challenging aspects of gene therapy development and industrialization processes and provide stable, efficient, and economical rAAV Fast Services to accelerate gene and cell therapy development efforts from discovery phase to commercialization.
Related News
Sangamo Therapeutics Secures Accelerated Approval Pathway for Gene Therapy in Fabry Disease
Sangamo Therapeutics has announced a major advancement in its gene therapy program for Fabry disease, as the U.S. FDA has provided a clear pathway for Accelerated Approval. This decision could potentially speed up approval timelines by three years, with a Biologics...
[2024/10/18] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
GSK sues Moderna over mRNA vaccine patents, seeks ‘reasonable royalty’
GSK on Tuesday unveiled a lawsuit filed against Moderna in Delaware federal court, alleging that its patented inventions provide the “foundation” for Moderna’s mRNA vaccine portfolio. GSK said it’s looking to recover “a reasonable royalty” for Moderna’s tens of...
Gene Therapy Automatically Converts Omega-6 to Omega-3 Fatty Acids in the Body
Shriners Children's Develops New Technology to Prevent Childhood Obesity ST. LOUIS, Oct. 16, 2024 /PRNewswire/ -- According to the Centers for Disease Control, nearly 20% of children and teens are considered obese. Research shows it can have a dramatic impact on a...
Related Services

AAV Packaging - NHP Grade
READ MORE

Off the Shelf AAV Products
We offer a wide catalogue of pre-made AAVs for a multitude of research needs.
READ MORE

AAV Capsid Engineering
Proven technology paving your path to effective therapies for cancer or genetic disorder
READ MORE

