An experimental “decoy” has provided long-term protection from infection by the pandemic virus in mice, a new study finds.
Led by researchers at NYU Grossman School of Medicine, the work is based on how the virus that causes COVID-19, SARS-CoV-2, uses its spike protein to attach to a protein on the surface of the cells that line human lungs. Once attached to this cell surface protein, called angiotensin converting enzyme 2 (ACE2), the virus spike pulls the cell close, enabling the virus to enter the cell and hijack its machinery to make viral copies.
Earlier in the pandemic, pharmaceutical companies designed monoclonal antibodies to glom onto the spike and neutralize the virus. Treatment of patients soon after infection was successful in preventing hospitalization and death. However the virus rapidly evolved through random genetic changes (mutations) that altered the spike’s shape enough to evade even combinations of therapeutic monoclonal antibodies. Thus, such antibodies, which neutralized early variants, became about 300 times less effective against more recent delta and omicron variants.
Published online this week in the Proceedings of the National Academy of Sciences, the study describes an alternative approach from which the virus cannot escape. It employs a version of ACE2, the surface protein to which the virus attaches, which unlike the natural, cell-bound version, is untethered from the cell surface. The free-floating “decoy” binds to the virus by its spikes so that it can no longer attach to ACE2 on cells in airways. Unlike the monoclonal antibodies, which are shaped to interfere with a certain spike shape, the decoy mimics the spike’s main target, and the virus cannot easily evolve away from binding to ACE2 and still invade cells.
Treatment with the decoy, either by injection or droplets in the nose, protected 100% of the study mice when they were infected in the lab with an otherwise lethal dose of SARS-CoV-2. The decoy lowered the virus load in the mice 100,000-fold, while mice exposed to a non-active control treatment died. Decoy treatment of mice that were already infected with SARS-CoV-2 caused a rapid drop in viral levels and return to health. This suggests that the decoy could be effective as a therapy post-infection, similar to monoclonal antibodies, the researchers say.
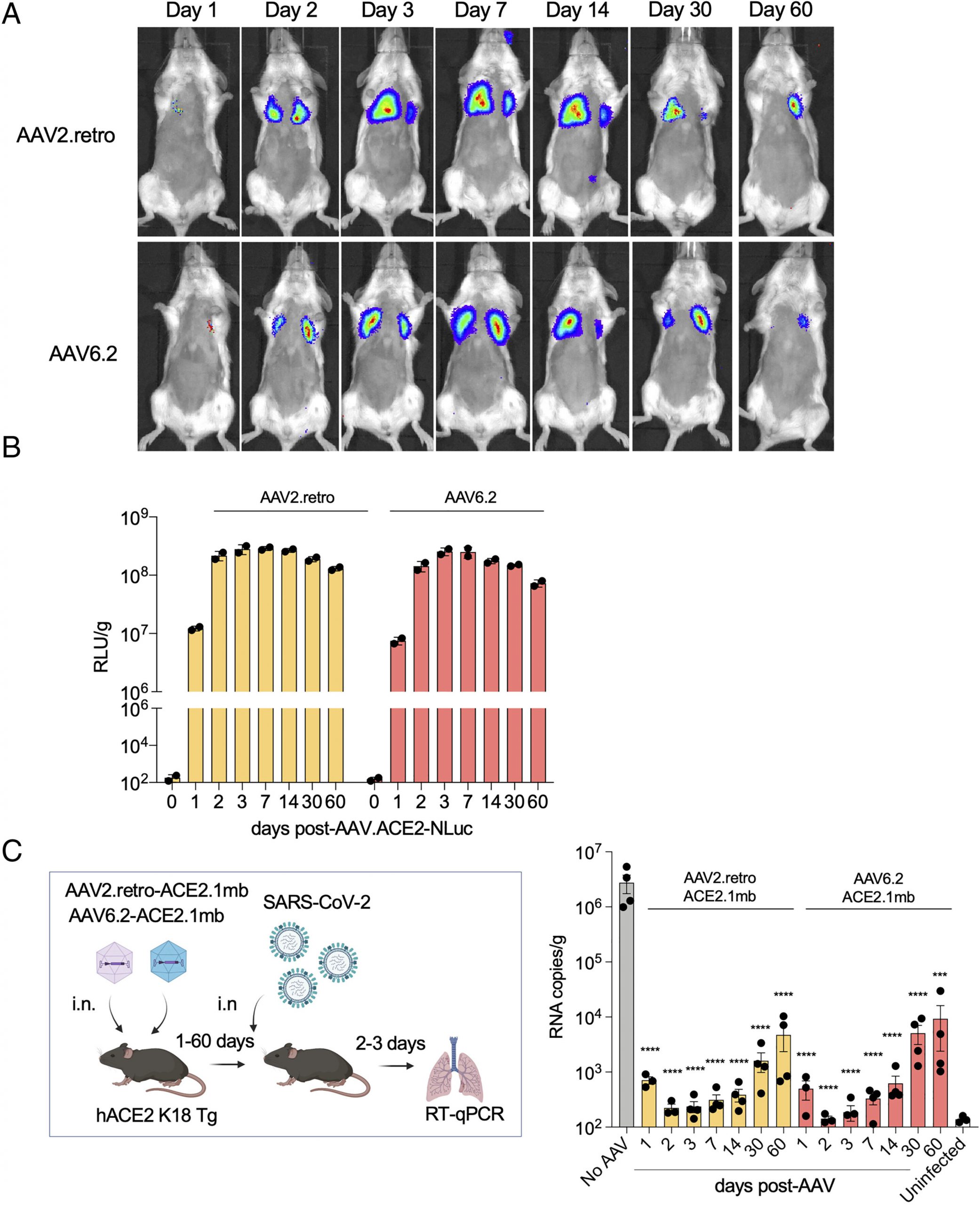
“What is remarkable about our study is that we delivered the decoy using a harmless, adeno-associated virus or AAV vector, a type of gene therapy that has been found in previous studies to be safe for use in humans,” says senior study author Nathanial Landau, Ph.D., professor in the Department of Microbiology at NYU Langone Health. “The viral vector instructs cells in the body to produce the decoy so that the mouse or person is protected long-term, without the need for continual treatment.” Administered the vector, says Landau, the treatment caused cells not only to make the decoy, but to continue making it for several months, and potentially for years.
Importantly, vaccines traditionally include harmless parts of a virus they are meant to protect against, which trigger a protective immune response should a person later be exposed. Vaccines are less effective, however, if a person’s immune system has been compromised by diseases like cancer, or in transplant patients treated with drugs that suppress the immune response to vaccination. Decoy approaches could be very valuable for immunocompromised patients globally, adds Landau.
Future pandemics
For the new study, the research team made key changes to a free ACE2 receptor molecule, and then fused the spike-binding part of it to the tail end of an antibody with the goal of strengthening its antiviral effect. Attaching ACE2 to the antibody fragment to form what the team calls an “ACE2 microbody” increases the time that the molecule persists in tissues (its half-life). The combination also causes the molecules to form dimers, mirror-image molecular pairs that increase the strength with which the decoy attaches to the viral spike.
Whether administered via injection into muscle, or through droplets in the nasal cavity, the study’s AAV vectors provided mice with long-lasting protection COVID infection, including the current omicron variants.
The approach promises to be effective even if another coronavirus, a type of virus common in birds and bats or apes, were to be transferred to humans in the future, an event termed “zoonosis.” As long as the future virus also uses ACE2 to target cells, the decoy would be ready for “off-the-shelf” soon after an outbreak. If the virus were to somehow switch its receptor a different protein on the surface of lung cells, the decoy could be modified to target the new virus, says Landau.
Along with Landau, the study authors were Takuya Tada and Julia Minnee in the Department of Microbiology at NYU Grossman School of Medicine.
More information: Takuya Tada et al, Vectored immunoprophylaxis and treatment of SARS-CoV-2 infection in a preclinical model, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2303509120
Journal information: Proceedings of the National Academy of Sciences
From Medical Express: https://medicalxpress.com/news/2023-05-experimental-decoy-sars-cov-infection.html
PackGene is a CRO & CDMO technology company that specializes in packaging recombinant adeno-associated virus (rAAV) vectors. Since its establishment in 2014, PackGene has been a leader in the AAV vector CRO service field, providing tens of thousands of custom batches of AAV samples to customers in over 20 countries. PackGene offers a one-stop CMC solution for the early development, pre-clinical development, clinical trials, and drug approval of rAAV vector drugs for cell and gene therapy (CGT) companies that is fast, cost-effective, high-quality, and scalable. Additionally, the company provides compliant services for the GMP-scale production of AAVs and plasmids for pharmaceutical companies, utilizing five technology platforms, including the π-Alpha™ 293 cell AAV high-yield platform and the π-Omega™ plasmid high-yield platform. PackGene’s mission is to make gene therapy affordable and accelerate the launch of innovative gene drugs. The company aims to simplify the challenging aspects of gene therapy development and industrialization processes and provide stable, efficient, and economical rAAV Fast Services to accelerate gene and cell therapy development efforts from discovery phase to commercialization.
Related News
Sangamo Therapeutics Secures Accelerated Approval Pathway for Gene Therapy in Fabry Disease
Sangamo Therapeutics has announced a major advancement in its gene therapy program for Fabry disease, as the U.S. FDA has provided a clear pathway for Accelerated Approval. This decision could potentially speed up approval timelines by three years, with a Biologics...
[2024/10/18] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
GSK sues Moderna over mRNA vaccine patents, seeks ‘reasonable royalty’
GSK on Tuesday unveiled a lawsuit filed against Moderna in Delaware federal court, alleging that its patented inventions provide the “foundation” for Moderna’s mRNA vaccine portfolio. GSK said it’s looking to recover “a reasonable royalty” for Moderna’s tens of...
Gene Therapy Automatically Converts Omega-6 to Omega-3 Fatty Acids in the Body
Shriners Children's Develops New Technology to Prevent Childhood Obesity ST. LOUIS, Oct. 16, 2024 /PRNewswire/ -- According to the Centers for Disease Control, nearly 20% of children and teens are considered obese. Research shows it can have a dramatic impact on a...
Related Services

AAV Packaging - NHP Grade
READ MORE

Off the Shelf AAV Products
We offer a wide catalogue of pre-made AAVs for a multitude of research needs.
READ MORE

AAV Capsid Engineering
Proven technology paving your path to effective therapies for cancer or genetic disorder
READ MORE

