
GMP Plasmid DNA Manufacturing Platform
- Production Capacity: 2 GMP Plasmid DNA production Lines
- Comprehensive QC and Analytical Services
- Production Scale: 5L-200L
GMP AAV Manufacturing Platform
- Production Capacity: 4 GMP AAV production lines
- Production Scale: 25L-500L
- Production Yield: up to 10^17 vg/batch
- Production Platform: Adherent / Suspension


Analytical Development & QC Platform
- Analytical Development: Based on project-specific methodologies
- Established 40+ Analytical Testing Methods including TCID50, rcAAV and AUC
- Analytical Verification and Validation: Risk-Based and Phase Appropriate
- Batch Release and In-process Testing
- Stability Studies
Quality Assurance
- Single-use Technology
- Compliant with NMPA/FDA/EMA regulations
- Unidirectional Flow, Physical segregation System
- Phase Appropriate Compliance solution

Process Development Capabilities
QbD Process Optimization with a total PD+AD lab of 5000+ sqft
Process Development Services
- Cell line development, adaptation and cell banking (GMP)
- Medium screening and cell culture optimization
- Upstream bioreactor process optimization
- Harvest process optimization
- Purification process optimization
- Scalability assessment
- Improved target product profile
- Facility fit and technology transfer to GMP manufacturing
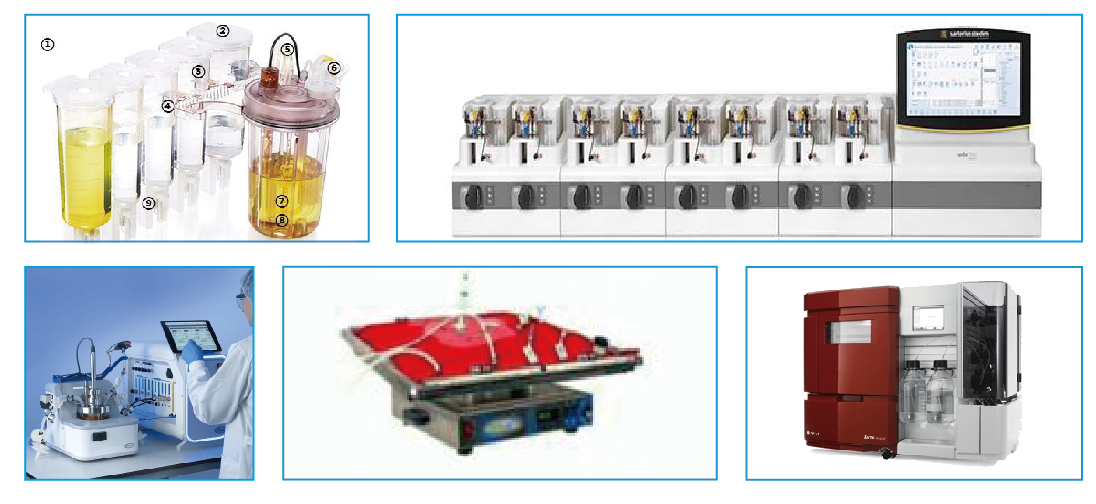
Current PD Capabilities
- iCells nano bioreactors
- Wave bioreactors: 5L, 25L
- Sartorius amber 250 high throughput bioreactors system
- Applikon 3L, 15L
- AKTA Avant, AKTA pure
- Solution prep up to 200L
In-House Analytical Capabilities
Full panel analytical capability serving AAV and DNA plasmid release and product characterization; 40+ analytical methods established and qualified.
| Category | Test | Method |
|---|---|---|
| Identity | AAV Capsid | SDS PAGE/HPLC-MS |
| Purity | Protein Purity | SDS PAGE/HPLC |
| UV Purity | A260/A280 | |
| Empty capsids | AEX HPLC | |
| AUC | ||
| Potency | Capsid Titer | ELISA |
| Genome Titer | ddPCR | |
| Infectious Titer | TCID50 | |
| In Vitro Bioactivity | Cell-based assay | |
| Impurity | Residual hcDNA | qPCR, ddPCR |
| hcDNA size distribution | CE/ddPCR | |
| Residual E1A | ddPCR | |
| Residual HCP | ELISA | |
| Residual BSA | ELISA | |
| Residual Plasmid | ddPCR | |
| Residual Nuclease | ELISA | |
| Residual Iodixanol | HPLC | |
| Residual Ligand | ELISA | |
| Safety | Mycoplasma | qPCR |
| rcAAV | Cell Assay+qPCR | |
| Bacterial endotoxin | LAL | |
| Sterility | Direct Inoculation Method/Membrane filtration | |
| Adventitious agent | Cell Culture | |
| General Characterization | Appearance | Visual Inspection |
| Filling Volume | Minimum Filling Volume | |
| pH | pH Method | |
| Osmolarity | Osmometers | |
| Particulate matter | Light Obscuration Particle Count test | |
| Aggregation | Dynamic Light Scattering | |
| Poloxamer 188 Content | HPLC | |
| AAV Neutralizing antibody | ELISA / Cell-based assay |
CONTACT US
