A recent study conducted by researchers from the TIDU GENOV at the Institut du Cerveau in Paris, France, presents promising results for the treatment of Metachromatic Leukodystrophy (MLD), a severe neurodegenerative disease. Led by Emilie Audouard with key contributions from Françoise Piguet, the study was published in the June 2024 edition of Molecular Therapy: Methods & Clinical Development.
MLD is an autosomal recessive disorder triggered by mutations in the gene that encodes the enzyme arylsulfatase A (ARSA). Deficiency in ARSA leads to the accumulation of sulfatides, which progressively damages the myelin sheath, essential for nerve function, resulting in significant neurological deterioration.
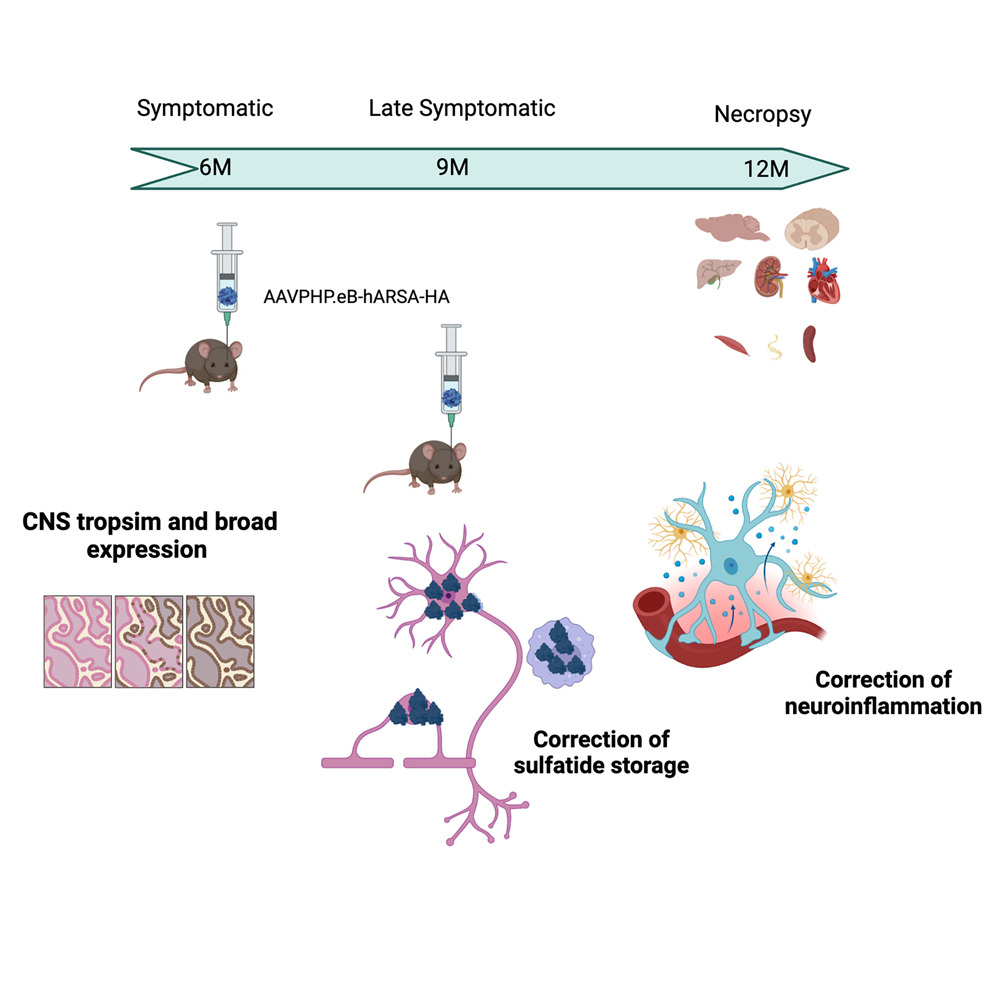
In their innovative study, the research team developed a gene therapy using an adeno-associated virus vector, AAVPHP.eB, to deliver a healthy ARSA gene directly into the bloodstream. This approach was tested in a mouse model genetically engineered to mimic human MLD.
The results were highly encouraging. Treated mice displayed significant reductions in sulfatide accumulation and neuroinflammation, critical markers of MLD. The therapy was effective at various doses and stages of the disease, indicating its potential adaptability for clinical use. Notably, the treatment successfully crossed the blood-brain barrier, a notable achievement since many neurological treatments fail at this hurdle.
These findings are particularly relevant as they offer a potential treatment pathway for symptomatic MLD patients, who currently have limited options once the disease manifests. The capability of the intravenous gene therapy to mitigate disease markers in a mouse model opens the door to Phase I/II clinical trials in humans, marking a significant stride towards a viable treatment.
The next steps involve scaling this study to larger animal models to confirm safety and efficacy on a broader scale before moving to human trials. This research not only paves the way for new MLD treatments but also illuminates potential therapeutic avenues for other lysosomal storage disorders.
This breakthrough study highlights the potential of gene therapy to transform the treatment landscape for patients suffering from MLD and similar genetic disorders. By bypassing previous treatment limitations, such as the blood-brain barrier, it offers hope for effective interventions in conditions where none existed before.
The full research details are available in Molecular Therapy: Methods & Clinical Development, providing an in-depth look at the methodology and results that could soon lead to new treatment protocols for devastating diseases like MLD.
https://www.cell.com/molecular-therapy-family/methods/fulltext/S2329-0501(24)00064-0
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
Sangamo Therapeutics Secures Accelerated Approval Pathway for Gene Therapy in Fabry Disease
Sangamo Therapeutics has announced a major advancement in its gene therapy program for Fabry disease, as the U.S. FDA has provided a clear pathway for Accelerated Approval. This decision could potentially speed up approval timelines by three years, with a Biologics...
[2024/10/18] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
GSK sues Moderna over mRNA vaccine patents, seeks ‘reasonable royalty’
GSK on Tuesday unveiled a lawsuit filed against Moderna in Delaware federal court, alleging that its patented inventions provide the “foundation” for Moderna’s mRNA vaccine portfolio. GSK said it’s looking to recover “a reasonable royalty” for Moderna’s tens of...
Gene Therapy Automatically Converts Omega-6 to Omega-3 Fatty Acids in the Body
Shriners Children's Develops New Technology to Prevent Childhood Obesity ST. LOUIS, Oct. 16, 2024 /PRNewswire/ -- According to the Centers for Disease Control, nearly 20% of children and teens are considered obese. Research shows it can have a dramatic impact on a...
Related Services

Plasmids GMP Services

AAV GMP Services


