Some are saying that mRNA medicines—mRNA vaccines and mRNA therapeutics alike—will open a golden era of medicine. And the enthusiasm is catching. Following the precedent set by the first mRNA-based COVID-19 vaccines, hundreds of new mRNA medicines are moving through development pipelines. More than 300 disease indications are being targeted.
The unprecedented speed, versatility, and effectiveness of mRNA vaccines has prompted a rapid expansion of the technology targeting many more infectious diseases, as well as new treatments that fight cancer and treatments that replace proteins that are absent or dysfunctional due to genetic mutations.
To learn more about the current status and near-term potential of mRNA medicines, GEN invited several leaders in the field to share their perspectives. They responded by highlighting the field’s current challenges and most promising solutions. Most important, they shared their predictions about the field’s exciting future.
Successes and gaps
The initial mRNA-based vaccines for targeting SARS-CoV-2 undoubtedly represented a “stellar achievement,” says Helen O. McCarthy, PhD, professor of nanomedicine, School of Pharmacy, Queen’s University Belfast. “[They] demonstrated that the human immune system could be trained to recognize and mount an immune response for preventing infection or reducing the symptoms. This has led to a pipeline of prophylactic vaccines for other diseases of concern which includes, but is not limited to, influenza, respiratory syncytial virus, Zika, Nipah, monkeypox, malaria, and HIV. This illustrates the impact that mRNA technology is having.”
According to McCarthy, the clinical pipeline looks very promising for a number of other applications: “Clinical mRNA trials also include a host of other indications. They include asthma, cystic fibrosis, chronic obstructive pulmonary disease, muscular dystrophies, and epidermolysis bullosa. All of these really demonstrate the opportunities with this technology.”
However, McCarthy points out that some significant challenges remain. One example she cites is delivery. “The delivery system … must ensure that the mRNA is shuttled inside the cell,” she explains. “That is, mRNA translation into vaccine antigens or therapeutic proteins happens only after cytoplasmic delivery. For this to happen, the mRNA must be packed into nanoparticles that fall within a range capable of entering the cell, predominantly via endocytosis. To date, the lipid nanoparticle (LNP) technologies have been tremendously successful in doing this, but there is always room to improve.”
McCarthy’s research focuses on the use of peptides to deliver nucleic acids. “These peptides are composed of natural amino acids and are designed to condense the mRNA into nanoparticles that enter cells and escape endosomes without the cell recognizing this as foreign,” she details. “This enables multiple administrations without provoking antipeptide antibodies. The peptide nanoparticles do not circulate for long periods of time and enter cells quickly.”
According to McCarthy, the use of peptides has several other important advantages: “The peptides are not restricted as to the size of nucleic acid with which they can complex, as this is based upon charge. Furthermore, they can be lyophilized and stored at room temperature.”
There are other applications of this technology. “Apart from both prophylactic and therapeutic vaccines, we are also incorporating these nanoparticles into injectable hydrogels for bone repair, along with 3D-printed and electrospun devices for wound healing,” McCarthy relates. “Our current work is particularly focused on a novel targeted delivery platform for mRNA.”
Inhaled therapeutics
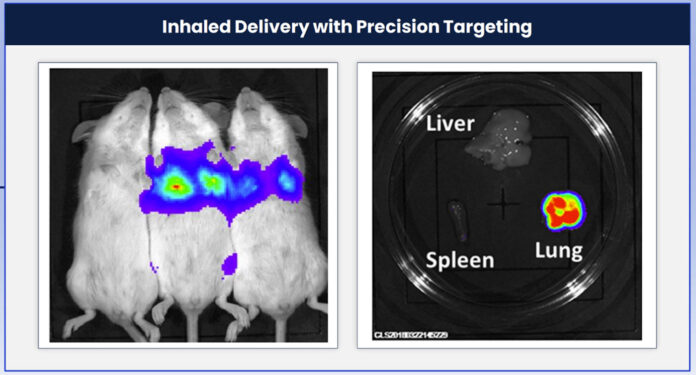
“Beyond vaccines, we’re now looking toward mRNA’s use in therapeutics and opening additional routes of delivery beyond intravenous administration, including nebulization and nasal sprays for inhaled delivery,” reports Carsten Rudolph, PhD, the CEO of Ethris. “In the near future, inhaled mRNA therapies have the potential to treat chronic or genetic diseases of the lung, such as chronic obstructive pulmonary disease, primary ciliary dyskinesia, and pulmonary alveolar proteinosis.”
However, Rudolph points out that the field still has problems that need solutions, several of which are being pursued by Ethris. “Despite the potential of inhaled mRNA therapeutics, the mechanical and thermal instability of LNP-delivered mRNA therapeutics presents a challenge,” he says. “When creating these medicines, we knew, right off the bat, that we had to overcome the challenge of LNP aggregation during nebulization. Such aggregation can hinder delivery, potentially lengthening application times, triggering immune responses, and jeopardizing treatment effectiveness. Another challenge is mRNA’s inherent immunogenicity, potentially causing unwanted inflammation or responses against the therapy. Yet another challenge is the vulnerability of mRNA itself to degradation.”
According to Rudolph, Ethris has developed a suite of proprietary technology platforms for improving the precision of delivery, the stability of RNA, and the efficacy of treatment. He adds, “We’ve also made a number of improvements in the manufacturing process for mRNA medicines.”
Specifically, Ethris’ SNaP lipidoid LNP platform tackles therapeutic potency and delivery of mRNA by better retention of the therapeutic payload locally at the site of administration. “The therapies have precise exposure at the target site,” Rudolph asserts. “Unlike current commercial mRNA vaccines, they don’t show up where they are not desired, that is, in tissues such as the liver, heart, and brain.”
“[The company’s stabilizer technology] allows for extended room temperature storage and handling of mRNA medicines,” he continues. “Additionally, our SNIM RNA technology conquers mRNA’s inherent instability and immunity barriers, with greatly increased tolerability to unmodified mRNA. Notably, it can be repeatedly administered, leading to consistent production of therapeutic proteins within the body.”
Ethris has begun a Phase I study of ETH47 for the treatment and prophylaxis of respiratory viral infections. The company is also applying its inhaled technology in rare lung diseases including primary ciliary dyskinesia, whose symptoms resemble cystic fibrosis and for which there are currently no approved therapies.
Immunomodulation and beyond
Although vaccines against infectious diseases remain prominent on the mRNA therapeutics radar, other applications are making headway. “Early personalized cancer vaccine data from several RNA companies raises some hopes, although this needs to be confirmed,” says Gilles Besin, PhD, the CSO of Orbital Therapeutics. “Certainly, in vivo treatment using chimeric antigen receptor (CAR) T cells represents an immediate application that can unlock the therapeutic potential of RNA. However, the question of delivery to immune cells is still unresolved. Progress has been made and has encouraged the biotechnology world to exert more effort.”
Other advancements in cancer therapeutics include the mRNA-driven production of functional proteins to either control tumor progression or modulate (that is, boost) immune responses. Further, mRNA-encoded proteins for genome editing may be able to disrupt tumor survival genes and provide enhanced efficacy of traditional cancer therapies.
Expanding beyond cancer, mRNA-based therapeutics may also play an important role in the in vivo production of functional proteins that are absent or nonfunctional due to genetic mutations. Besin points out that durability is especially important in this area: “Durable expression allows for less frequent injection and eases the dosing regimens. This could change the life of many patients.”
Besin reports that Orbital’s expression enhancing platform utilizes a closed RNA molecule for increased resistance to exonucleases. “Such circular RNA,” he says, “can extend durability of RNA expression in a cell-dependent manner. In addition, in the vaccine context, circular RNA has been shown to elicit greater T-cell responses.” The company is currently conducting preclinical studies.
Second-generation advances
“The beauty of mRNA technology is that it can continuously be improved,” says Alexander Zehnder, MD, the CEO of CureVac. “Luckily, we are just at the beginning of what’s possible with mRNA.”
Zehnder agrees that delivery of mRNA therapeutics remains a key area where improvement is needed: “As a field, we need to continue to advance LNP technology, which is used for mRNA delivery, to increase the potency, safety, and stability of mRNA therapeutics. Additionally, to expand mRNA’s potential across a broad range of possible indications, advancing LNPs to achieve more precision in tissue targeting will be critical.”
In parallel with delivery, mRNA stability remains another key issue. RNA is a relatively large and inherently unstable molecule prone to degradation by 5¢ and 3¢ exonucleases and endonucleases. Thus, different strategies are being investigated to improve its stability in the delivery process. For example, CureVac customizes the 5¢ and 3¢ untranslated regions and the open reading frames of mRNAs to engineer for greater stability and thus increased translation into protein product.
“We believe our technology platform is a broad and versatile platform to develop optimized mRNA-based medicines with the potential for a differentiated profile in terms of tolerability, stability, and protein expression,” Zehnder elaborates. “We are currently advancing our second-generation mRNA backbone in prophylactic vaccines as well as oncology.
“This backbone features targeted optimizations for improved intracellular mRNA translation for early and strong immune responses at low doses. In collaboration with GSK, we generated early, positive data from our COVID-19 and seasonal flu mRNA vaccine trials in 2023 to provide validation for our second-generation mRNA technology.”
CureVac is testing two mRNA-based COVID-19 vaccine candidates in a Phase II study and announced positive results in January. In addition, the company recently began testing an influenza vaccine candidate in another Phase II study. The candidate performed well in an initial trial, and data is expected in 2024.
Zehnder predicts a bright future for mRNA therapeutics: “Its speed, versatility, and ultimate potential to help patients is a powerhouse combination that will propel mRNA medicines forward.”
Fast translation
“We aim to cut down research time by offering deep insights into the RNA landscape and providing the granular data behind the holistic trends,” says Ioana Panait, a senior RNA research analyst at Beacon, a science database solution for RNA developers. Panait points out that incredible achievements have been made in the RNA field in 2023, and that the fastest-growing part of this field is mRNA therapeutics.
“The data highlights a 29% growth of the preclinical space and 32% growth of the clinical space of mRNA therapeutics in development throughout 2023,” she details. “Over the past year, approximately 250 new mRNA therapies have entered pipelines, while at the same time showing a fast translation into clinical stages.”
Painit expects the field to continue growing: “The potential of mRNA therapeutics is highlighted not only by the number of new therapies being developed or by drug developers entering the field, but also by the diversity of disease indications investigated. Over 300 different disease indications are currently being studied in the space.”
Although Panait also cites delivery of mRNA therapeutics as a challenge needing to be addressed, she indicates some practices are slowing overall progress: “There is an increase of proprietary delivery systems, which can indicate movement in the right direction; however, developers continue to disclose these delivery systems at later stages of drug development, which will contribute to this challenge being harder to overcome. There also is a lack of pharmacokinetic/pharmacodynamic data in the mRNA field, with less than 5% of drugs having preclinical pharmacokinetic/pharmacodynamic data released publicly.”
Despite the remaining challenges, Panait believes that there is cause for great optimism. “The expansion of the mRNA field is no longer surprising,” she declares. “The field is bound to continue growing at a similar pace in the coming years.”
https://www.genengnews.com/topics/omics/rna-medicines-address-stability-and-deliverability-challenges/

Check out our mRNA service to expedite your vaccine research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
Sangamo Therapeutics Secures Accelerated Approval Pathway for Gene Therapy in Fabry Disease
Sangamo Therapeutics has announced a major advancement in its gene therapy program for Fabry disease, as the U.S. FDA has provided a clear pathway for Accelerated Approval. This decision could potentially speed up approval timelines by three years, with a Biologics...
[2024/10/18] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
GSK sues Moderna over mRNA vaccine patents, seeks ‘reasonable royalty’
GSK on Tuesday unveiled a lawsuit filed against Moderna in Delaware federal court, alleging that its patented inventions provide the “foundation” for Moderna’s mRNA vaccine portfolio. GSK said it’s looking to recover “a reasonable royalty” for Moderna’s tens of...
Gene Therapy Automatically Converts Omega-6 to Omega-3 Fatty Acids in the Body
Shriners Children's Develops New Technology to Prevent Childhood Obesity ST. LOUIS, Oct. 16, 2024 /PRNewswire/ -- According to the Centers for Disease Control, nearly 20% of children and teens are considered obese. Research shows it can have a dramatic impact on a...
Related Services

Plasmids GMP Services

AAV GMP Services


