“Unfortunately, this situation has hardly changed at all over the past two decades,” says Professor Klaus Strebhardt, Director of the Department of Molecular Gynecology and Obstetrics at University Hospital Frankfurt.
Of all ovarian cancer (high-grade) patients, 96% share the same clinical picture: The tumor suppressor gene p53 has mutated and is now non-functional. The gene contains the building instructions for an important protein that normally recognizes damage in the genetic material (DNA) of each cell. It then prevents these abnormal cells from proliferating and activates repair mechanisms that rectify the damage. If this fails, it induces cell death.
“In this way, p53 is very effective in preventing carcinogenesis,” explains Strebhardt. “But when it is mutated, this protective mechanism is eradicated.”
If a cell wants to produce a certain protein, it first makes a transcript of the gene containing the building instructions for it. Such transcripts are called mRNAs. In women with ovarian cancer, the p53 mRNAs are just as defective as the gene from which they were copied.
“We produced an mRNA in the laboratory that contained the blueprint for a normal, non-mutated p53 protein,” says Dr. Monika Raab from the Department of Molecular Gynecology and Obstetrics, who conducted many of the key experiments in the study, now published in Cancer Communications.
“We packed it into small lipid vesicles, known as liposomes, and then tested them first in cultures of various human cancer cell lines. The cells used the artificial mRNA to produce functional p53 protein.”
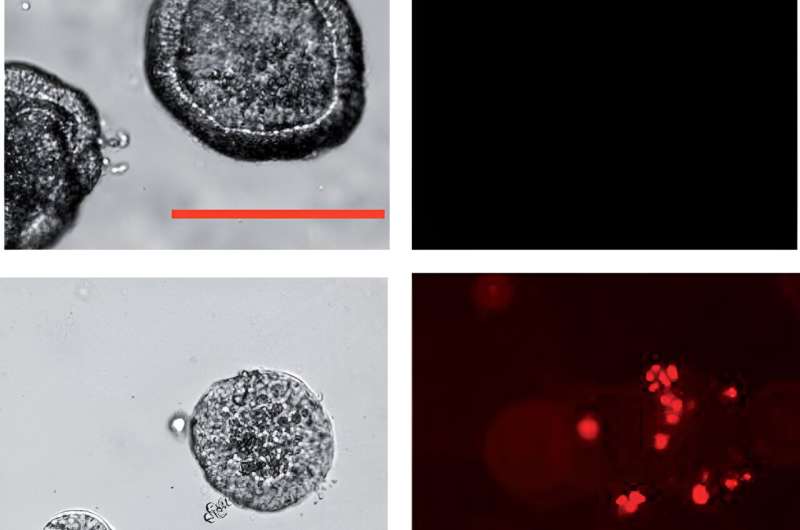
In the next step, the scientists cultivated ovarian tumors—organoids—from patient cells sourced by the team led by Professor Sven Becker, Director of the Women’s Clinic at University Hospital Frankfurt. After treatment with the artificial mRNA, the organoids shrank and began to die.
To test whether the artificial mRNA is also effective in organisms and can combat metastases in the abdomen, the researchers implanted human ovarian tumor cells into the ovaries of mice and injected the mRNA liposomes into the animals some time later.
The result was very convincing, says Strebhardt, “With the help of the artificial mRNA, cells in the animals treated produced large quantities of the functional p53 protein, and as a result both the tumors in the ovaries and the metastases disappeared almost completely.”
That the method was so successful is partly due to recent advances in mRNA technology. Normally, mRNA transcripts are very sensitive and degraded by cells within minutes. However, it is meanwhile possible to prevent this by specifically modifying the molecules. This extends their lifespan substantially, in this study to up to two weeks.
In addition, the chemical composition of the artificial mRNA is slightly different to that of its natural counterpart. This prevents the immune system from intervening after the molecule has been injected and from triggering inflammatory responses.
https://medicalxpress.com/news/2024-01-mrna-therapeutic-successfully-combats-ovarian.html

Check out our mRNA service to expedite your vaccine research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
Sangamo Therapeutics Secures Accelerated Approval Pathway for Gene Therapy in Fabry Disease
Sangamo Therapeutics has announced a major advancement in its gene therapy program for Fabry disease, as the U.S. FDA has provided a clear pathway for Accelerated Approval. This decision could potentially speed up approval timelines by three years, with a Biologics...
[2024/10/18] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
GSK sues Moderna over mRNA vaccine patents, seeks ‘reasonable royalty’
GSK on Tuesday unveiled a lawsuit filed against Moderna in Delaware federal court, alleging that its patented inventions provide the “foundation” for Moderna’s mRNA vaccine portfolio. GSK said it’s looking to recover “a reasonable royalty” for Moderna’s tens of...
Gene Therapy Automatically Converts Omega-6 to Omega-3 Fatty Acids in the Body
Shriners Children's Develops New Technology to Prevent Childhood Obesity ST. LOUIS, Oct. 16, 2024 /PRNewswire/ -- According to the Centers for Disease Control, nearly 20% of children and teens are considered obese. Research shows it can have a dramatic impact on a...
Related Services

Plasmids GMP Services

AAV GMP Services


